1. Introduction
Ehlers-Danlos syndrome (EDS) represents a heterogeneous group of heritable connective tissue disorders (CTD) characterized by variable combinations of hyperextensible skin, hypermobile joints, and tissue fragility. Based on obvious clinical presentations, Its prevalence is estimated as many as 1 in 5000 individuals (1), but more subtle laboratory testing probably increases the actual prevalence. At present, 7 EDS subtypes have been identified (2), but such a classification could probably be revised and enriched due to the complexity of the presently recognized genetic alterations. It is also possible (although not proven) that stem cells at the origin of fibroblasts are directly involved in the various and distinct molecular defects.
In recent years, some resemblances and similarities have been reported between EDS and a small set of apparently distinct CTD. The latter condition includes, for example, some cases of fibromyalgia (3), spontaneous preterm premature rupture of fetal membranes (4, 5), spontaneous cervical artery dissections (SCAD) (6-11), and spontaneous dissection of medium-size arteries (12, 13). Any of these disorders is characterized by specific alterations in the morphologic presentation and association of fibrillar collagen and elastic fibers.
SCAD is responsible for stroke among young and middle-aged adults. Gene mutations were identified only in rare SCAD cases without any obvious clinical sign of other heritable CTD (14-16). However, few patients with SCAD exhibit concurrent clinical and molecular manifestations of a clear-cut heritable CTD, including EDS of the vascular or classical type, Marfan syndrome, or osteogenesis imperfecta (9, 17). Moreover, some abnormal ultrastructural connective tissue alterations were present in the skin of these patients (6-11). Such findings suggested that a peculiar CTD leading to the weakness of the vascular walls and the subsequent spontaneous dissections.
We presently report a family with the proband suffering from SCAD, whose brothers and nephews showing complete or incomplete clinical signs of EDS hypermobile type (EDSH). All of them presented similar dermal ultrastructural changes. Such findings suggest that SCAD and EDSH are possibly related.
2. Case Presentation
A 41-year-old woman presented with a left vertebral arterial dissection. Incidentally, she was able to touch her nose tip with the tongue (positive Gorlin sign). In addition, she suffered from chronic lumbar pain and some tendon ruptures. Her cutaneous scars looked dystrophic and her joint hypermobility score in the Beighton scale reached 5 out of 9. Her three brothers and two nephews were examined. The 46-year-old brother suffered from multiple ankle sprains and tendinitis, but his Beighton scale level was zero. Her second 45-year-old brother presented with repeat episodes of ankle sprains and tendinitis, easy skin bruising, dystrophic scars and a positive Gorlin sign, but his Beighton scale remained at the 0 score. Her third 43-year-old brother exhibited a Gorlin sign and zero score at the Beighton scale.
Her second brother had two sons. The 18-year-old son presented with numerous tendon injuries, some fractures, 5 degrees genu valgum, easy bruising, dystrophic scars and a 4 out of 9 score in the Beighton scale. The second 15-year-old boy reached a 6 out of 9 score in the Beighton scale. He exhibited easy skin bruising and dystrophic scars. His clinical signs fitted the typical EDSH features.
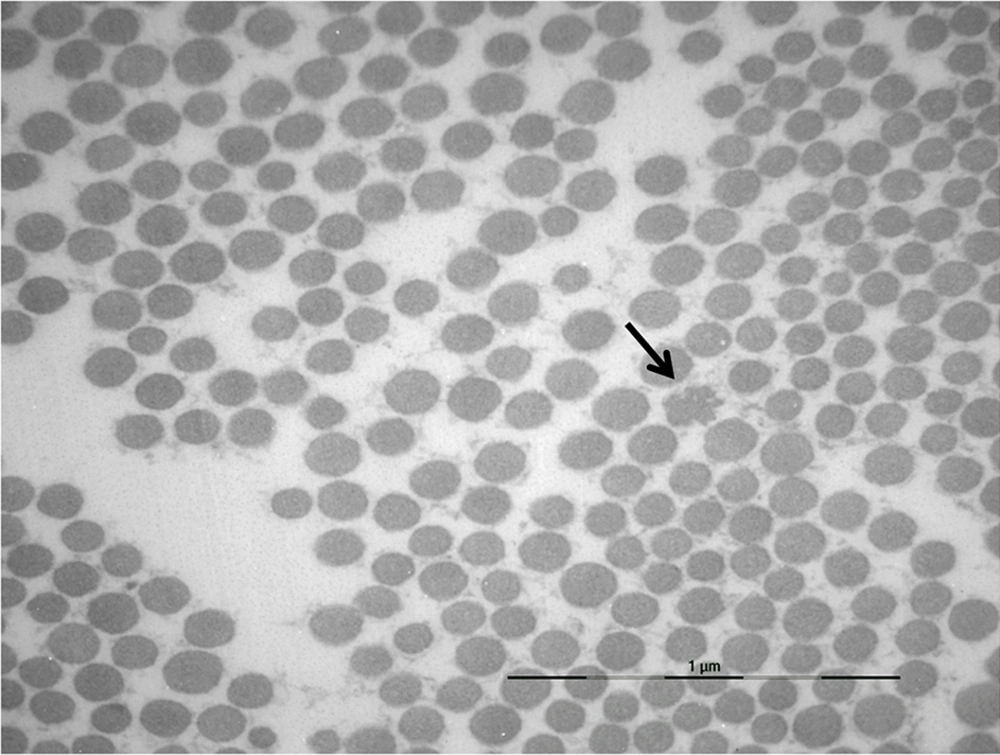
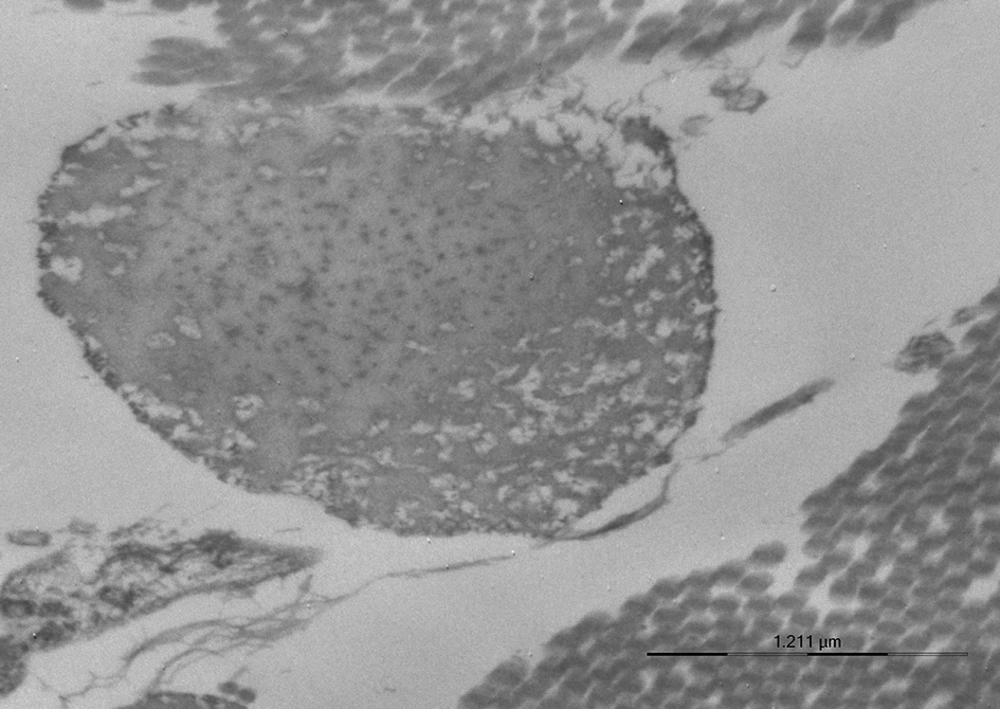
A skin biopsy was performed in the SCAD proband and her 5 family members. Ultrastructural examinations revealed collagen and elastic fiber changes similar to those reported in EDSH, namely collagen bundles with uneven fibril sizes, flower-like collagen fibrils, and irregularly enlarged interfibrillar gaps (Figure 1). Elastic fibers exhibited irregular contours (Figure 2). Such aspects were absent in control samples of skin of normal individuals.
3. Discussion
Joint hypermobility is clinically recognized when extension of the wrists and metacarpal phalanges brings the fingers parallel to the dorsal forearms, and when positive opposition brings the thumb to the flexor aspect of the forearm. In addition, the elbows and knees are possibly hyperextended, reaching in some instances more than 10 degrees. The flexion of the trunk is obtained with the knees fully extended and the hands flat on the floor. One point is given for each successful test, and the score of 5 out of 9 or greater defines hypermobility (2). Other complaints include deep pains of the arms and legs, flatfeet, odd gait, and lordotic posture.
EDSH is a relatively common condition affecting about 1 in 10,000 to 15,000 individuals (1). Predominant features include generalized joint hypermobility with recurrent dislocations and arthralgias. Musculoskeletal pain is a prominent feature. Some EDSH patients have dilatation or rupture of the ascending aorta, or both. Joint hypermobility as present in EDSH is expressed at a lesser extent in the lax ligament syndrome (18). Of note, this latter mild collagenopathy improves over time with the progressive increase in periarticular collagen fibril and bundle interconnections.
The 41-year-old woman presented with a left vertebral arterial dissection and some clinical EDSH features, including a 5 score in the Beighton scale, lumbar pains, tendon ruptures, dystrophic scars, and a Gorlin sign. Clinical signs of one of her nephews were similar to those reported in EDSH (1, 2). The other family members presented only minor diagnostic clues supporting but not firmly establishing the EDSH diagnosis. All of the family members exhibited ultrastructural dermal changes similar to those observed in EDSH (19, 20) and SCAD (6-9, 11).
Observation of this family with SEDH and SCAD signs suggests that both disorders are possibly related. In SEDH and SCAD, most gene mutations remain unsettled. The multisystemic manifestations observed in EDSH (21, 22), and SCAD possibly represents one of the manifestation of this disorder. The clinical presentations and ultrastructural aspects of the dermis are presently described in 6 members of a family. The features are those of SCAD and EDSH. These two conditions possibly represent closely related CTD suggesting a single condition with varied presentations.