1. Background
Atopic dermatitis, also known as atopic eczema, is one of the most common recurring skin diseases, typically associated with increased serum levels of immunoglobulin and a known personal and family history of the condition (1, 2). The prevalence of this disease is higher in children, affecting approximately 10% to 30%, and in adults, affecting 1% to 3%. The prevalence has been increasing in recent years, with higher rates observed in developed countries compared to developing countries (3, 4). The disease manifests with symptoms such as itching, typical eczema morphology, and dry skin, and may sometimes be accompanied by other symptoms such as keratosis pilaris, increased palm lines, ichthyosis vulgaris, conjunctivitis, cataracts, and perioral dermatitis (5).
The etiology and pathology of atopic dermatitis can be divided into three main stages: (1) Epidermal barrier dysfunction, (2) immune regulation disorder, and (3) changes in the skin microbiome, all of which are influenced by genetic regulation and environmental factors. Given the chronic and relapsing nature of eczema, various treatments such as antihistamines, topical steroids, and moisturizers are employed (6, 7). Antihistamines are used to alleviate itching and burning at the site of inflammation, while topical steroids, such as hydrocortisone and triamcinolone, are used to reduce redness, itching, and inflammation due to their immunomodulatory effects. Unfortunately, these medications often do not provide sufficient and definitive effectiveness, and their side effects remain a concern.
Considering these conditions, and given that the treatment of skin dermatitis is primarily symptomatic and long-term, the use of natural-origin drugs, such as extracts, essences, and vegetable oils, can potentially be considered as alternative medicine. These extracts, essential oils, and oils derived from various plants are often in liquid form and, in addition to possessing anti-inflammatory and anti-eczema properties, they also exhibit good skin permeability.
Calendula officinalis L., commonly known as marigold, from the Compositae family, is an annual plant found worldwide. Its topical applications can aid in healing wounds and soothing inflamed and damaged skin (8). The C. officinalis contains various phytochemical compounds, including sesquiterpene and flavonol glycosides, triterpenoid saponins, triterpene alcohols, flavonoids, carotenoids, xanthophylls, phenolic acids, and others such as sterols, mucilage, tocopherols, calendulin, bitters, phytosterols, resin, and volatile oil. Polyphenols and flavonoids are the main components responsible for its antioxidant and anti-inflammatory effects (9).
Fumaria parviflora L. (family Papaveraceae) is an herbaceous species (10). Its liquid form has been used externally for treating scabies, eczema, acne, and other skin disorders (11). Major chemical constituents include alkaloids like protopine and berberine, phenolic compounds, flavonoids, fumaric and ferulic acid, and sterols such as stigmasterol and campesterol. The antioxidant and anti-inflammatory effects of F. parviflora have been evaluated in experimental models, showing it can decrease cytokine levels such as TNFα, interleukin 1, and 6 (12).
Glycyrrhizaglabra Linn., commonly known as licorice, is from the Fabaceae family. Phytochemical screening of the G. glabra root revealed the presence of alkaloids, glycosides, carbohydrates, starches, phenolic compounds, flavonoids, proteins, pectin, mucilage, saponins, lipids, tannins, sterols, and steroids. The plant exhibits a wide range of pharmacological activities, including antimicrobial, antioxidant, anticancer, hypolipidemic, cardiovascular, central nervous, respiratory, immunological, anti-inflammatory, analgesic, and antipyretic effects (13, 14).
Curcuma longa L., commonly known as turmeric, from the Zingiberaceae family, is an herbaceous, evergreen plant. Curcumin, the main active ingredient of the rhizome, plays a key role in treating various pro-inflammatory chronic diseases. The C. longa contains about 3% to 6% phenolic content, including curcuminoids such as curcumin, dimethoxy curcumin, and bisdemethoxycurcumin. It has been reported to have antiarthritic, anti-inflammatory, antibacterial, antiallergic, and antioxidant effects (15).
2. Objectives
The present study aimed to compare the effect of a herbal cream formulation combining C. officinalis, G. glabra, C. longa, and F. parviflora with topical mometasone in the treatment of atopic dermatitis.
3. Methods
This randomized clinical trial was conducted from 2021 to 2022 among patients referred to dermatology clinics affiliated with Isfahan University of Medical Sciences at Al-Zahra and Sedighe Tahereh hospitals. Inclusion criteria were: (1) Age above 7 years, (2) a score of less than 50, (3) absence of physical illness that prevents receiving medication, (4) no treatment in the last 6 months, and (5) absence of drug or alcohol use in the last 6 months. Exclusion criteria included: (1) Pregnancy during the study, (2) failure to follow up with the patient, and (3) severe drug sensitivity.
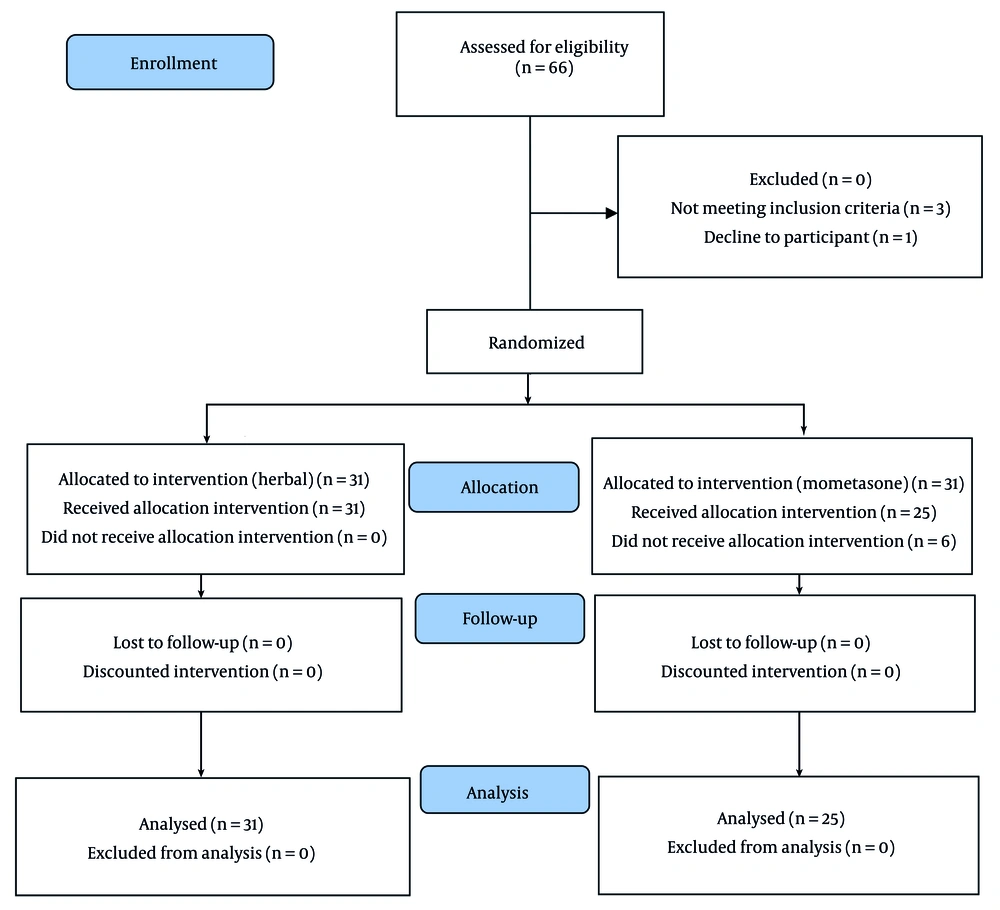
After considering the inclusion and exclusion criteria, participants were enrolled in the study in a completely random manner. The study method was fully explained, and written consent was obtained from all patients. The type of treatment was allocated randomly using a random number table (Figure 1). One group received a combined herbal medicine, while the other group received topical mometasone (manufactured by Aburihan Pharmaceutical Company). Each patient was placed into one of two groups of 31 individuals according to the time of entry. Both drug combinations were prepared in the same form by the associate doctor and given to the prescribing doctor, ensuring that the visiting therapist was blinded to the type of treatment.
Patients were treated with the medication daily for one month, and the medication was gradually tapered off the following month. The plants required for this study were purchased in coordination with reputable and approved perfumers. After approval by Dr. Zulfaqhari, a pharmacognosy expert at the Isfahan Faculty of Pharmacy, the plants were powdered using a grinder and prepared for extraction.
After extraction, all patients were visited by the attending physician on four occasions. All patients used the same type of soap for washing and wore cotton gloves during the treatment. The first visit involved receiving the medication and completing the SCORAD Questionnaire. The second visit occurred at the end of the fourth week to check for possible side effects. The third visit took place at the end of the eighth week to complete the second round of the questionnaire, and the fourth visit was at the end of the sixteenth week to check for side effects, assess the possibility of recurrence, and complete the third round of the questionnaires.
In both groups, the SCORAD scores were compared and analyzed to assess the response to treatment at weeks zero, eight, and sixteen. The SCORAD Index was used in this study for clinical evaluation (16). This index combines objective criteria (observable), such as the extent and severity of lesions, with subjective criteria (non-objective), such as daily itching and sleep loss. The extent of the lesion, ranging from 0 to 100, was calculated to measure the involved area using the Rule of Nines, and the results were collected.
The intensity of the lesion consists of six components: Redness (erythema), edema/papule, itching effect (excoriation), prominence of the stratum corneum of the epidermis (lichenification), serous secretion from the epidermis (oozing), or coagulated crusts (crusts), each scored from 0 to 3 points. The final index score is 103, calculated as follows: A/5 + 7B/2 + C, where A represents the extent of the lesion from 0 to 100, B represents lesion severity from 0 to 18, and C represents subjective symptoms from 0 to 20.
At the end of each visit, patients were asked to rate their level of satisfaction from 0 to 10 based on the Visual Analogue Scale (VAS) Index, with a score of 0 indicating the least satisfaction and 10 indicating the most satisfaction (17). This study was approved by the Ethics Committee of Isfahan University of Medical Sciences with the code IR.MUI.MED.REC.1400.379 and has also received the clinical trials registry code IRCT20211216053428N1.
Data analysis was conducted using SPSS software, version 24. Tables and graphs were utilized to describe qualitative variables, while central and dispersion indices were used for quantitative variables. An independent t-test was employed to analyze the data, with a significance level set at less than 0.05.
4. Results
Finally, after sampling, 25 individuals with an average age of 38.4 ± 14.02 years in the mometasone treatment group (including 7 men and 18 women) and 31 individuals with an average age of 42.74 ± 17.5 years in the herbal treatment group (including 11 men and 20 women) were included. In the herbal combination group, 24 individuals had hand eczema, 4 had leg eczema, 2 had trunk eczema, and 1 had facial eczema. In the mometasone group, 20 individuals had hand eczema, 2 had leg eczema, 2 had trunk eczema, and 1 had facial eczema. There was no significant difference between the two groups in terms of demographic information such as age and sex, as well as the size of the affected area, location of eczema, intensity of eczema, subjective symptoms, and the SCORAD Questionnaire score at the beginning of the study (P < 0.05).
The description of the results for the extent of the involved area, intensity of involvement, subjective symptoms, SCORAD Questionnaire, and VAS Questionnaire in the study population at the measured time intervals are shown in Table 1. As shown, the mean SCORAD Questionnaire score at the end of the 8th week of treatment (P = 0.003) and at the end of the 16th week of treatment (P = 0.005) was significantly higher in the group treated with herbal medicine compared to the group treated with mometasone. Additionally, the size of the affected area at the end of the 8th week of treatment (P = 0.024) and the end of the 16th week of treatment (P = 0.020) was significantly higher in the group treated with herbal medicine than in the group treated with mometasone. The severity of the affected area at the end of the 8th week of treatment (P = 0.029) and the end of the 16th week of treatment (P = 0.005) was also significantly higher in the group treated with herbal medicine than in the group treated with mometasone. Subjective symptoms at the end of the 8th week of treatment (P = 0.035) and the end of the 16th week of treatment (P = 0.032) were significantly higher in the group treated with herbal medicine compared to the group treated with mometasone.
| Factors | Herbal Treatment | Mometasone Treatment | P-Value b |
|---|---|---|---|
| Extent of the involved area week 0 | 24.19 ± 7.31 | 24.40 ± 6.66 | 0.913 |
| The extent of the involved area week 8 | 14.19 ± 9.92 | 7.60 ± 11.28 | 0.024 |
| The extent of the involved area week 16 | 14.03 ± 10.03 | 7.20 ± 11.18 | 0.020 |
| Subjective symptoms week 0 | 3.45 ± 3.12 | 2.80 ± 2.16 | 0.380 |
| Subjective symptoms week 8 | 2.32 ± 2.56 | 1.00 ± 1.84 | 0.035 |
| Subjective symptoms week 16 | 2.16 ± 2.66 | .80 ± 1.75 | 0.032 |
| Intensity of the involved area week 0 | 6.57 ± 1.94 | 6.12 ± 2.14 | 0.422 |
| Intensity of the involved area week 8 | 3.87 ± 2.39 | 2.32 ± 2.79 | 0.029 |
| Intensity of the involved area week 16 | 3.71 ± 2.39 | 1.88 ± 2.29 | 0.005 |
| SCORAD score week 0 | 30.11 ± 7.88 | 27.43 ± 7.69 | 0.206 |
| SCORAD score week 8 | 16.71 ± 12.00 | 5.3 ± 9.92 | 0.003 |
| SCORAD score week 16 | 16.81 ± 11.18 | 8.18 ± 10.18 | 0.005 |
| VAS score week 8 | 6.26 ± 3.17 | 8.00 ± 3.04 | 0.042 |
| VAS score week 16 | 6.58 ± 2.97 | 8.00 ± 3.04 | 0.085 |
Abbreviation: VAS, Visual Analogue Scale.
a Values are expressed as mean ± SD.
b Independent sample t-test.
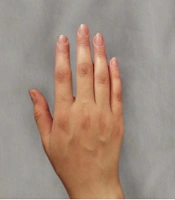
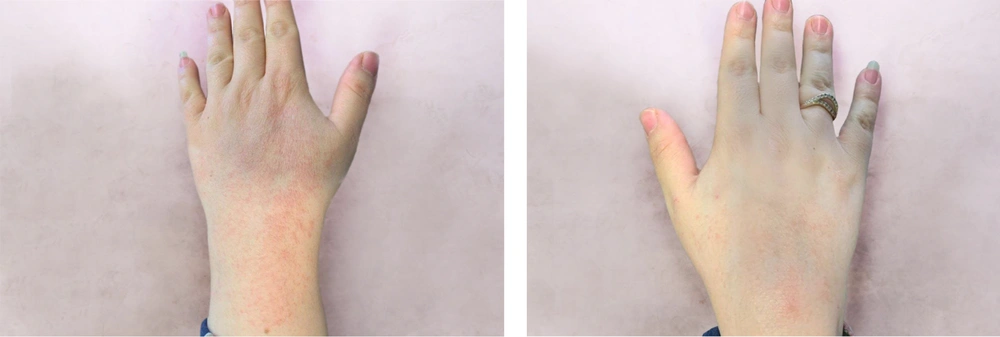
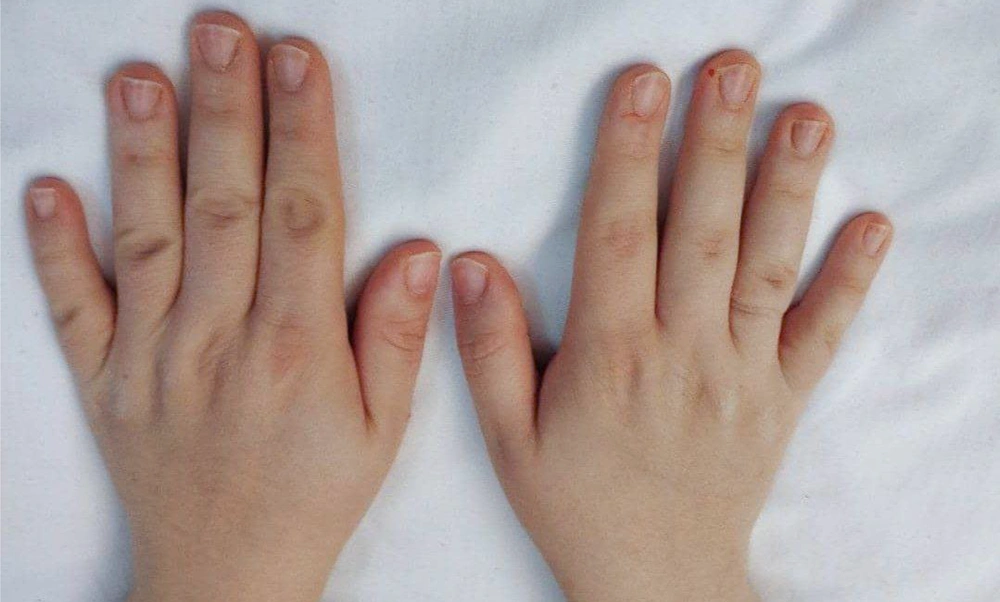
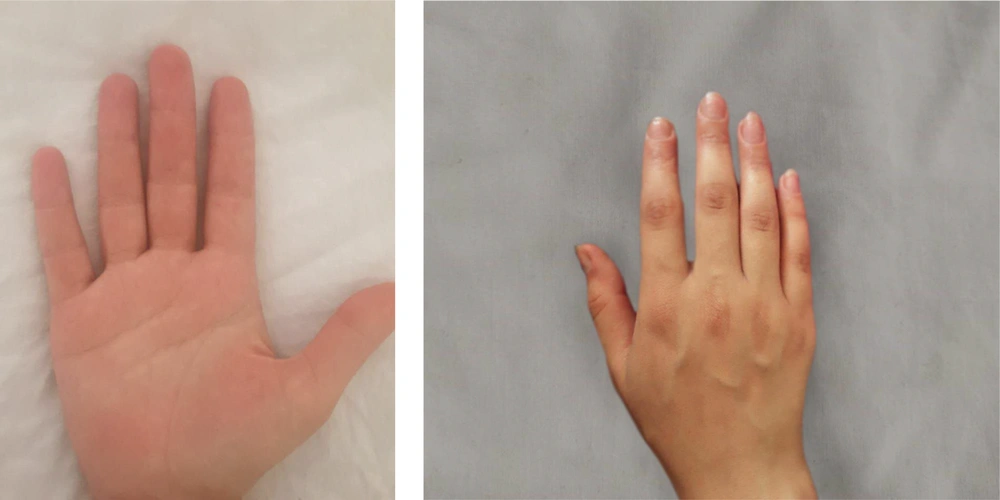
The group treated with herbal medicine reported significantly higher pain levels than the mometasone group at week 8 (P = 0.042), but this difference was not significant at week 16 (P = 0.085). There was no relationship between the therapeutic response in the herbal combination group and the mometasone group with the site of involvement (P < 0.05). Comparing the effectiveness of each treatment method within the group, as shown in Table 2, the results of our study indicated that both mometasone and herbal combination treatments were effective in treating patients (P < 0.001). No treatment complications were observed in any of the patients. Picture samples of the patients' hands before and after treatment with the herbal combination and mometasone are shown in Figures 2 - 5.
| Medications and Factors | P-Value a |
|---|---|
| Mometasone treatment | |
| The extent of the involved area week 0 vs. extent of the involved area week 8 | 0.000 |
| The extent of the involved area week 0 vs. extent of the involved area week 16 | 0.000 |
| Intensity of the involved area week 0 vs. intensity of the involved area week 8 | 0.000 |
| Intensity of the involved area week 0 vs. intensity of the involved area week 16 | 0.000 |
| Subjective symptoms week 0 vs. subjective symptoms week 8 | 0.000 |
| Subjective symptoms week 0 vs. subjective symptoms week 16 | 0.000 |
| SCORAD score before-SCORAD score W8 | 0.000 |
| SCORAD score before-SCORAD score after | 0.000 |
| Herbal treatment | |
| The extent of the involved area week 0 vs. extent of the involved area week 8 | 0.000 |
| The extent of the involved area week 0 vs. extent of the involved area week 16 | 0.000 |
| Intensity of the involved area week 0 vs. intensity of the involved area week 8 | 0.000 |
| Intensity of the involved area week 0 vs. intensity of the involved area week 16 | 0.000 |
| Subjective symptoms week 0 vs. subjective symptoms week 8 | 0.000 |
| Subjective symptoms week 0 vs. subjective symptoms week 16 | 0.000 |
| SCORAD score week 0 vs. SCORAD score week 8 | 0.000 |
| SCORAD score week 0 vs. SCORAD score week 16 | 0.000 |
a Paired sample t-test.
5. Discussion
The indigenous traditional approach to treating various disorders is now widely accepted. A significant number of drugs on the market are either directly derived from herbal resources or have been developed through structural modification. Natural products have substantially contributed to the development of new drugs and formulations (18, 19). Chronic inflammatory skin diseases, including atopic dermatitis, remain major health problems for the global population. Due to the reversibility of the disease and the numerous skin problems it causes over time, it significantly affects patients' quality of life (20). Despite understanding the various factors involved in the disease, a definitive cure for atopic dermatitis has not yet been found.
This clinical trial aimed to compare the effectiveness of a herbal cream formulation combining C. officinalis, G. glabra, C. longa, and F. parviflora. In this study, the SCORAD Questionnaire was used to evaluate the response to treatment, and the VAS Questionnaire was used to assess patient satisfaction and pain levels. The results showed that both mometasone and the herbal combination were effective in treating patients. However, the group treated with mometasone demonstrated significantly better results in both treatment response rate and patient satisfaction rate at weeks 8 and 16.
Therefore, it can be concluded that while the herbal combination provided a good therapeutic response in patients with atopic dermatitis, it was less effective than mometasone. This may be attributed to the use of mometasone, a corticosteroid with average potency, in the control group. Another reason could be the low dosage of the herbal medicine, as participants applied the compound only once daily to the target area. Although various topical herbal products for eczema containing C. officinalis, C. longa, or G. glabra individually are available on the market, we propose using all four together to harness their cumulative anti-inflammatory and anti-eczema effects.
Another study by Iraji et al. compared the effectiveness of F. officinalis and Silymarin with mometasone for the treatment of eczema. Their results indicated that the reduction in the SCORAD score was significant in both groups, but no significant difference was observed between them (21). The difference in results can be attributed to the different ingredients used.
Our study was not without limitations. The most important limitation was the small size of our study population, and it is recommended that future studies involve a larger population. Additionally, due to the uncomplicated nature of the herbal compounds used in our study, future research should focus on using these drugs in higher doses and for a longer duration to determine their long-term results and effectiveness.
5.1. Conclusions
In conclusion, our study confirms the acceptable effectiveness of both mometasone and the herbal combination in treating atopic dermatitis. However, it is noteworthy that the herbal combination demonstrated significantly lower efficacy compared to mometasone. Given that the safety and efficacy of these medicinal plants and their topical uses have been proven in many studies, we suggest designing more clinical trials for cream formulations to enhance the safety and efficacy of these products.