1. Context
The term apoptosis is the death of cells via self-immolation. An alternative definition of apoptosis is the programmed cell death (PCD) or cell immolation. Apoptosis process is energy dependent and self-controlled and can occur either in a single cell or a group of cells.
All along the initial stages of apoptosis, pyknosis, and cell shrinkage are detectable via light microscopy. The mass of cells is teeny by cell shrinkage; the cytoplasm is condensing (the most diagnostic aspect of apoptosis). Apoptotic bodies called budding are formed via karyorrhexis and division of cell fragments. Finally, phagocytic action is performed by macrophages, parenchymal cells, or neoplastic cells on these bodies (1).
Reduction of connection between caspases and intracellular ATP changes a continuous apoptotic procedure to a necrotic procedure (2). Inflammatory reaction is the most significant difference between apoptosis and necrosis; no inflammatory reaction occurs during the process of apoptosis since:
1- Cellular components are not released by apoptotic cells into the environment.
2- Encircling cells quickly phagocytize the apoptotic buddies.
3- Anti-inflammatory cytokines are not produced by engulfing cells (1).
The two vital apoptotic pathways are the intrinsic or mitochondrial pathway and the extrinsic or death receptor pathway. Moreover, an alternative pathway requires T-cell-mediated cytotoxicity and perforin-granzyme-dependent cell-immolation. Apoptosis occurs via either granzyme A or B in the perforin-granzyme pathway. All these pathways except granzyme A are lead to activation of caspase 3 and result in DNA fragmentation, degradation of nuclear protein and cytoskeletal, crosslinking of protein, formation of apoptotic bodies, expression of ligands for phagocytic cell receptors, and in the end, are phagocytized by phagocytic cells (3).
2. Importance of Apoptosis in Hair and Skin
2.1. Role of Apoptosis in Disease Treatment
2.1.1. Acne Vulgaris
One of the most prevalent inflammatory skin diseases is acne vulgaris that involves more than 80% of pre-adulthood population of the developed countries. Four vital factors in the pathogenesis of acne are: the increase of sebum production, hypercolonization and formation of biofilm by Propionibacterium acnes (P. acnes), the increase of acro-infundibular keratinocyte (KC) proliferation with comedo formation, and follicular and perifollicular inflammation (4).
The p53 controls a series of pivotal genes associated with acne pathogenesis such as AR, FoxO transcription factors, BLIMP1, and mTORC1, which all play an important role in acne pathogenesis as well as pharmacological responses of anti-acne agents (5). The p53, a genome protector, is a particular regulator for cell homeostasis. It regulates the most crucial cellular reactions such as AR signaling and IGF-1, and via induction of MDM2 discharges p53-induced cellular reactions by means of ubiquitination and proteasomal degradation of p53, FoxO1, and FoxO3a, accordingly (6).
2.1.2. Wound Healing
Wound healing is an elaborated procedure that requires soluble mediators, extracellular matrix, blood cells, and parenchymal cells. Wound healing consists of three stages: (1) inflammatory reactions, (2) new tissue formation, and (3) tissue restoration (7). In the initial stages of tissue restoration, after 12 hours wound injured inflammatory cells undergo apoptosis initiation process (8). A well-marked feature of initiating the event of the corneal wound healing reaction is stromal keratocyte apoptosis (9).
Releasing growth signals via apoptotic cells trigger stem cell proliferation via caspases 3 and 7 proteases requiring the caspase-mediated activation of phospholipase A2 and the posterior production and release of the lipid signal prostaglandin E2, a cell proliferation stimulant (10).
The apoptosis of immune cells can end the inflammation and start the healing, and phagocytosing the apoptotic cells by macrophages is a major key of the phenomenon related to actively tissue restoration from wound inflammation (11).
2.1.3. Psoriasis
Psoriasis is a prevalent skin condition distinguished by a hyperplasia and the imperfect differentiation of epidermal KCs. Overexpression of Bcl-XL occurs in all layers of epidermis in psoriatic condition (12).
Complex interactions among the innate immune system, dendritic cells, and genetically activated T-cells predispose abnormal KCs spread psoriasis. Psoriasis also occurs due to the infiltration of activated T-lymphocytes in the epidermis and dermis resulting in the production of several inflammatory cytokines such as interferon (IFN)-α, interleukin (IL)-2, and tumor necrosis factor (TNF)-α (13) moreover, in vitro studies indicate that resistance of psoriatic KCs to apoptosis is more than that of normal skin KCs (14).
Ultraviolet (UV) radiation (311 nm) can be used to induce apoptosis in psoriatic KC for treatment procedure. Apoptosis can be directly induced by UVB in T-cells, and it can be also indirectly induced by upregulation of FasL in KC (15).
UVB phototherapy can treat the disease by eliminating T-cells or KCs via apoptosis, or immune modulation by IL-10 (released from KC) (16).
2.1.4. Vitiligo
Selective destruction of melanocytes responsible for skin pigmentation causes a skin disorder called vitiligo. Clinical signs of vitiligo are the localized or generalized white macules on the skin and progression of the disease is commonly unpredictable. Selective destruction of melanocytes can occur by necrosis or apoptosis (17). Some investigations show that apoptosis is a more probable process (18).
Induction of apoptosis is caused by certain factors such as suppressing the expression of BCL2 or increasing the expression of p53 such as the UVB. Some studies indicate that T-cells and macrophages are located nearby the residual perilesional melanocytes in generalized vitiligo. Accordingly, it is likely that apoptosis of melanocytes in vivo occurs in vitiligo by auto-reactive T-cell and/or macrophages (19).
2.1.5. Skin Tumor
Skin tumors cover malignant melanoma and nonmelanom cancers (NMSCs) including epithelial source neoplasms such as keratoacanthoma (KA), squamous cell carcinoma (SCC), and basal cell carcinoma (BCC) (20).
The mutation of the p53 gene results in the loss of the defensive role and occurrence of pro-oncogenic actions leading to uncontrolled cell proliferation and cell resistance to apoptosis. Consequently, resistance to apoptosis is a crucial fact in photocarcinogenesis, and the removal of cells that contain extra UV-induced DNA damage is a pivotal means to defend against skin cancer development. The tumor suppressor protein, P53, can affect both main pathways of apoptosis, intrinsic and extrinsic (21).
Failure of lymphocytes infiltrated to the tumor to mediate and regulate tumor growth is related to signaling malfunction, impulsive apoptosis of circulating and tumor-infiltrating T-lymphocytes (22).
Tumor signals can resist against different apoptotic signals (23). CTLs (CD8+ T-lymphocytes) might bypass tumor resistance to Fas/FasL-induced death via granzyme-mediated apoptosis (24).
Capability of granzyme B to skip caspase cascade and to stimulate the apoptotic pathways at different access points is remarkable; ability of tumors to evolve various defenses in the caspase cascade and intracellular signaling lead to their resistance to apoptotic stimulators (25). In vitro studies investigated that granzyme B/perforin pathway can induce apoptosis in multiple-drug-resistant (MDR) and death-receptor resistant cell lines (24), although another study indicates that overexpression of the protease inhibitor PI-9/SPI-6 (serpin) can lead to the resistance of tumors against granzyme-mediated killing (26). Downregulation of Fas expression on the cell surface can survive SCC from Fas/FasL-mediated apoptosis (27), and regulate apoptosis of infiltrating T-lymphocytes by expressing FasL on their surfaces (22, 23). Anyway, KAs constantly express high levels of Fas in association with inflammatory cells sensitive to T-cell mediated apoptosis leading to tumor suppression (27).
2.1.6. Skin Pigmentation
The skin color of humans varies from extremely fair/light to extremely dark depending on a racial/ethnic background, but the density of melanocytes in a given area (e g the back or arms) is almost the same on any skin type. KCs in fair skin lead to the accumulation of poorly pigmented melanosomes above the nuclei, though in dark skin the considerably pigmented melanosomes develop separately in KCs, therefore, maximizing their absorption of light. The melanocytes of epidermis proliferate slowly, if at all, under normal condition, and are actually resistant to apoptosis for their high expression of Bcl2 (28). The density and differentiation of melanocyte are influenced by the surrounding, including UV and factors exuded by adjacent fibroblasts and KCs (29). There are some recognizable differences including reposition of melanin, protection against DNA damage, and induction of apoptosis in melanin-containing KCs. Translocation of melanin from the lower epidermis upward is stimulated by UV radiation more often in dark skin (30). Also, UV-induced apoptosis apparently occurs in dark skin rather than fair skin that indicates a more effective elimination of UV-damaged cells; this may cause reduction in photocarcinogenesis of darker skin (31).
Under normal condition, p53 in the KC is a skin tanning signal transducer and a vital factor in regulation of KC-melanocyte signaling cycle and skin pigmentation. Under normal conditions, p53 is the transducer of the skin tanning signal and a vital factor in regulation of KC-melanocyte signaling cycle and skin pigmentation in the KC. When genetic disruption of melanin biosynthesis occurs in white colored skin, this cycle acts as a stimulator of melanocyte proliferation. The p53 was intended in the current study as a pivotal tumor suppressor and a lot of mechanisms by which the p53 pathway may be dysregulated in tumors define its significance as a tumor suppressor (32).
2.1.7. Unwanted Hair Removal
Physical methods to remove unwanted hair growth (light-induced hair removal and laser) are based on inducing catagen and apoptosis in the hair follicle (HF) (33). These methods provide the selective absorption of light by the HF chromophores such as melanin.
2.2. Role of Apoptosis In Disease Induction
2.2.1. Sun Burn
UVR is an active apoptosis inducer in the skin. Apoptotic epidermal KCs, known as sunburn cells (SBCs), have some pyknotic nuclei and a shrunken, eosinophilic cytoplasm in their look. SBCs can be detected within eight hours after exposure to UVR at maximum dosage for 24 - 48 hours and fade away by 60 - 72 hours (34).
Pyrimidine (6 - 4) pyrimidine photoproducts (64PP) and cyclobutene pyrimidine dimers (CPD), which occur at a ratio that is different from 4:1 to 10:1, are the most noticeable photo- lesions induced by UVB that exert potent mutagenic effect (35).
UVB-induced apoptosis involves some different signaling pathways such as: (1) direct DNA-damage that causes apoptosis; (2) death receptor-mediated apoptosis; and (3) apoptosis induced by the formation of reactive oxygen species (ROS) (36).
Nucleotide excision repair (NER) eliminates a large number of DNA lesions removing short single-strand DNA nucleotide stretches (24 - 32 bases) surrounding the disrupted site, and then altering the removed DNA lesion (37). SBC numbers are increased in patients with xeroderma pigmentosum; a condition in which genetic defects debilitate the NER process (38). The pivotal role of DNA damage in apoptosis induced by UVB- confirmed by in vivo investigations in humans-indicates that the increase of DNA repair by the topical use of repair enzymes reduces the SBCs formation (39).
Genes that regulate the apoptotic pathway and the ones associated with age-related dysfunction are responsible for forming SBCs. UV activates the p53 and p21, induces p53-independent KC apoptosis, apparently by the membrane activated signal transduction pathways (40). UV-induced KC apoptosis is blocked when BCL2 is over expressed or pretreated with tumor-promoting phorbol ester (41). UV-induced JunB expression, loss of c-Jun expression, and accelerated UV-induced KC apoptosis increase in AP-1 immune reactivity over all the epidermal cell layers that result in increasing the number of apoptotic KCs suggest the role of these proteins in UV-induced apoptosis (40).
2.2.2. Leishmaniasis
Leishmania major, L. tropica, and L. aethiopica cause cutaneous leishmaniasis (CL) in the old-world and close histological studies can clarify the inflammation patterns in these lesions (42).
In the dermis, which comprises ulcers caused by L. major, macrophages are infected by L. major and infiltrate neutrophils, monocytes, plasma cells, and activate T-cells. Proliferation and activation of KC with upregulation of activation markers such as HLA-DR are mentioned together with signed epidermal hyperplasia, necrosis, and apoptosis (43). Also, upregulation of Fas and FasL can be observed in the skin in L. aethiopica- and L. major-induced CL and ulcers containing blocked apoptotic KCs.
In CL caused by L. major, TRAIL as well as the pro-apoptotic receptor TRAIL-R2 and the pro-inflammatory receptor TRAIL-4 were upregulated in infected cases compared to healthy controls (44).
2.2.3. Graft-Versus-Host Disease
Graft-versus-host disease (GVHD) can be caused by allogeneic bone-marrow transplantation and it is the result of tissue damage caused by cytotoxic T-cell. KCs in cutaneous GVHD die in apoptotic process (45) mediated by secreted TNF-α and FasL presented by lymphocytes. FasL inhibition or lack of perforin reduces mortality and cutaneous GVHD manifestation (46). It is important to neutralize both FasL and TNF-α with antagonizing antibodies to completely repeal this disorder (47).
2.2.4. Toxic Epidermal Necrolysis (TEN, the Lyell Syndrome)
Toxic epidermal necrolysis (TEN, the Lyell syndrome) and the Stevens-Johnson syndrome (SJS) are infrequent acute dermatological diseases described by mucosal erosions and epidermal necrosis (48) with the severity of contact reduction between dermis and epidermis and enormous apoptosis of KCs (49), and FasL expression is increased KCs of lesioned skin in patients with TEN (50).
By incubating the skin cryosections of patients with TEN, Fas-sensitive Jurkat cells undergo Fas-dependent apoptosis (51). In addition, intravenous administration of human immunoglobulin taken from healthy blood donors that contains anti-Fas antibodies is a promising therapy to treat the patients with TEN (52). These results imply the significant role of the Fas signaling pathway in TEN development.
2.2.5. Erythema Multiforme Major
EM, both major and minor variants, is dominant due to previous infections, especially with herpes simplex virus (HSV), and only rarely with medications.
EM major differs from TEN and SJS in terms of severity; regarding the distribution and pattern of the skin lesions in both major and minor EM, Fas-mediated apoptosis may play a role in EM pathogenesis as the pathogenesis of TEN and SJS (50, 53). The simultaneous overexpression of epidermal Fas antigen indicates that apoptosis of KCs may be a part of the pathophysiology of all these conditions.
The intense expression of Bcl-2 along the epidermal basal layer and in the mononuclear cells of the dermis infiltrates both EM and SJS/TEN. The product of the proto-oncogene BCL-2, i e, Bcl-2 protein, blocks apoptosis in various conditions and is supposed to inhibit a fundamental step in the pathway of apoptotic cell death (54).
2.2.6. Pemiphagus
Pemphigus is an autoimmune cutaneous blistering disease defined by the autoantibodies presence against structural proteins of the intracellular junctions. Pemphigus foliaceus (PF) and pemphigus vulgaris (PV) are the two leading types. In PF, the target for exposure to IgG is desmosome cadherin desmoglein-1 (dsg-1), while in PV, antibodies to desmoglein-3 (dsg-3) in mucosal PV and to dsg-1 and dsg-3 in mucocutaneous PV lead to loss of adhesion in the suprabasal layer (55, 56).
Apoptotic KCs exist in PV lesioned tissue. When PV-IgG presents in KCs, Bcl-2 expression is reduced, FasL is secreted, and various apoptotic proteins are upregulated. There is a possibility of participation of the extrinsic apoptotic pathway in PV. Activation of p38 mitogen-activated protein kinase (MAPK) as a required event for PV IgG induced acantholysis (57).
2.2.7. Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is one of the systemic autoimmune diseases characterized by the autoantibodies presence to nuclear and cytoplasmic antigens in conjunction with a broad range of clinical signs. Photosensitivity is one of the SLE properties. The aggregation of apoptotic cells due to decreased removal of apoptotic cells, an increased rate of apoptosis, or a combination of both is considered a significant factor in the extension of inflammatory lesions, as observed in the skin (58, 59).
There was an increase in the expression of Fas in KCs in LE lesions, while the expression of Bcl-2 decreased after UVB irradiation, and an apoptosis induction was observed in the skin of patients with SLE (60).
More pro-inflammatory reaction occurs after UVB irradiation related to apoptotic cells. It is assumed that this might be induced by the opsonization of apoptotic KCs by autoantibodies and afterwards clearance by macrophages and/or other phagocytes.
2.2.8. Dermatitis Herpetiformis
Dermatitis herpetiformis (DH) is an autoimmune sub-epidermal blistering disease defined by chronic and recurrent eruptions of erythematous, popular, urticarial, vesicular, and bullous lesions. Granular IgA deposits at the dermal papillae express the immunological marker of the disease precisely correlated with a gluten-sensitive enteropathy (GSE), indistinguishable from coeliac disease (CD) (61). A genetic background (HLA-DQ2, -DQ8) appears to allow an immune hyperactivity, which causes specific reactions to humoral and cellular immunity (62). Also, some investigations show that Th2-lymphocytes play a significant role in the DH immune inflammation (63).
There were considerable apoptotic cells in the basal layer of epidermis in DH lesions; in addition. hyperactivation of the two main pathways of apoptosis, i e, mitochondrial (by intracellular expression of Bax and Bcl-2) and death receptor (by expression of Fas and FasL on cell surface) pathways were observed in DH. FasL binding to Fas induces intracellular signals that finally lead to caspases activation and result in nuclear fragmentation and beginning the apoptotic cell death (64). Epidermal Fas expression can be significantly unregulated by various stimuli such as cytokines (Il-1, TNF-a, IL-15 and IFN-y), UV radiation, and viral infection (65).
2.2.9. Eczematous
The term eczematous dermatitis (eczema) includes several inflammatory skin diseases including atopic dermatitis that are often defined by the vesicles formation associated with exudation. KC apoptosis is the initiating event in the development of the epidermal pathology observed in eczematous dermatitis (66).
The clinical symptoms include papules, redness, itching, vesicles, and scaling. Since T-cells form the majority of cellular infiltration in the eczematous dermatitis, a dysregulated, cytokine mediated response of the immune system seems to be a significant pathogenic factor. KCs within eczematous lesions exhibit abnormal expression of MHC class II antigens (HLA-DR), ICAM-1 (CD54), and IFN-g persuadable protein-10, all of which are intensely induced by IFN-g (67). Type 2 cytokines (IL-5, IL-4) and type 1 cytokine (IFN-g) play important roles in the inflammation of skin (68). Skin-infiltrating T-cells cause Fas-induced KC apoptosis, which is an important mechanism in the eczematous dermatitis pathogenesis. In addition to the interaction of FasL-FasR in eczematous dermatitis, it may be possible that activated T-cells use granule-mediated killing by granzymes and perforin (69).
2.2.10. Lichen Planus
Apoptosis is also involved in lichenoid tissue. Reactions are histologically characterized by basal KC damage and mononuclear cell infiltrate at the derma epidermal junction. The individual KCs damaged, termed as colloid (civatte or cytoid) bodies, on histopathological examination of the lesions. These colloid bodies are apoptotic cells. Cytotoxic T-cells and natural killer cells mediate the mode of cell death in lichenoid tissue reaction. Cytotoxic T-cells release granzyme A and B (70).
2.2.11. Diabetic Wound
The transcription factor, nuclear factor-erythroid 2--related factor 2 (NRF2), regulates the adaptive response to exogenous and endogenous oxidative stress (71), as well as cell migration, apoptosis, proliferation, and differentiation (72).
The protective role of NRF2 and the potential therapeutic effect of NRF2 activators are demonstrated in a diabetic nephropathy animal model. Skin tissue is demonstrated by greater oxidative DNA damage, apoptosis, and compensatory NRF2 pathway activation. Studies further confirmed that pharmacological NRF2 activation contributes to important events of wound healing, including oxidative stress attenuation, promotion of proliferation and migration, and decreased apoptosis under high glucose. Oxidative stress is prevalent in diabetes. Excessive oxidative stress damages proteins, lipids, and DNA in the cells, which may ultimately lead to cell death and subsequent tissue dysfunction. In response to oxidative stress, cells activate NRF2 to repair the damage. Therefore, pharmacological NRF2 activation ensures that the cells are protected (less damage, less apoptosis) and it also regulates the expression of other proteins that play significant roles in wound healing (MMP9, TGF-b, and migration- and proliferation-related genes) through direct or indirect mechanisms; some of which, as well as the effects of different oxidative stress levels on NRF2 signaling/wound healing, remain to be clarified in future studies (73).
2.2.12. Androgenic Alopecia
Anagen phase of the follicular cycle results in androgenic alopecia (AGA). Progressive anagen truncation leads to miniaturized follicles, and eventually, follicular loss is associated with gentle inflammation, but not scarring.
Androgens such as dihydrotestosterone (DHT) plays pivotal role in the development of androgenetic alopecia (AGA), but the mechanism of this effect is not understood yet. Therefore, due to the important role of apoptosis in normal hair cycle, it can be concluded that the expression of apoptosis is related to immune histochemical markers in scalp biopsies and is different in the individuals clinically affected by AGA (74).
Bcl‐2 and PCNA (proliferating cell nuclear antigen) are considerably expressed in epidermal basal layer and follicular dermal papilla (P < 0.001). Elevated expression of Bcl‐2 is observed in perifollicular lymphocytic penetration of the bald area. TUNEL staining shows no apoptotic KCs in the hair follicles indicating that proapoptotic Bax and Fas were not expressed, but in the epidermis. Follicular miniaturization occurs as a result of constant perifollicular inflammatory infiltrate resulting in the expression of anti-apoptotic Bcl‐2 in dermal lymphocytes and reduction of proliferation rates in the bald area of patients (75).
2.2.13. Skin Aging
Thinning of the dermis and subcutaneous adipose layer, flattening of the dermal epidermal junction, and decreased numbers of melanocytes, KCs, Langerhans cells, and mast cells are vital aspects of sun-protected aged skin. The thickness of epidermis varied and the number of Langerhans cells also decreased, but increased numbers of melanocytes are indicative of photodamaged skin. Mitogens inhibit DNA synthesis that results in irreversibly arrest growth of G1 phase (76). In aged skin, both intrinsic (chronology) and extrinsic (the photoaging) pathways of apoptosis play key roles in aging of the skin (77).
Also, some early response genes such as c-myc, c-jun, and Ha-ras are expressed via aging cells, though cfos or the Id genes are not expressed. Phosphorylation of retinoblastoma protein (Rb) and late G1 gene expression inhibit (76, 78) downregulation of cyclin A and BCL-2 family, glucocorticoid receptor, androgen receptor, epidermal growth factor receptor, and IL-2 receptor, expression of Rb, p53, and p21Waf-1/cip; all inhibitors of cell cycle progression proceed apoptosis in the epidermis. P21 plays the role of p53 in its absence, and senescent and quiescent cells produced it in significant amounts (79).
AP-1 transcription factor family genes are dysregulated during aging and play a significance role in terminal differentiation and apoptosis. In addition, C-fos is downregulated in senescent cells, whereas continuous expression of c-fos induces cell death in the epidermis (80).
Therefore, it is understood that senescence of the epidermal KC is a specific mechanism and is not similar to either differentiation or apoptosis.
2.3. Role of Apoptosis in Skin and Hair Cells
2.3.1. UVB and Fibroblast Apoptosis
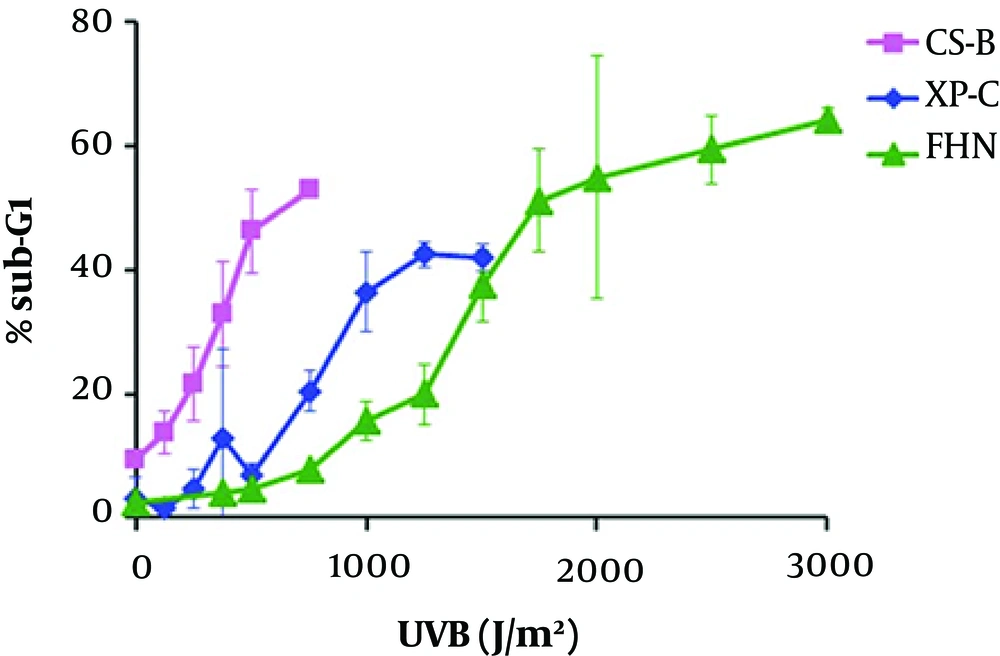
Transcription-coupled repair (TCR)-deficient human fibroblasts induce apoptosis by p53 accumulation at more reduced UV doses than cells capable in NER sub-pathway; thus, indicating that lesions present in the transcribed regions of the genome act as signals for the stabilization of p53 and apoptosis (81, 82). In comparison to normal fibroblasts, global genome repair (GGR)-deficient (XP-C) fibroblasts present a similar dose-response in relation to the accumulation of p53 and RNA synthesis (81). However, according the studies with primary human fibroblasts and CHO (Chinese hamster ovary) cells, there is a correlation between UV-induced apoptosis and cell progression through the S (synthesis) phase of cell cycle (83).
Mdm2 is a transcriptional target of p53. The absence of induction, in human fibroblasts deficient for TCR was associated with apoptosis after UV (84); thus compatible with the negative role of Mdm2 in the p53 function. It can be realized that Mdm2 induction would be dependent on the UV dose; in fact, regulation and interaction of p53 and Mdm2 are dose-dependent in human fibroblasts (85).
The apoptosis rates for normal (FHN), TCR-deficient (CS-B) primary human fibroblasts, and GGR-deficient (XP-C) were determined after cell radiation (majority in G1 phase) with increasing doses of UVB, by flow cytometry quantification: According to Figure 1, for the three cell lines, the apoptosis levels reached a plateau; increasing doses of UVB cause apoptosis at saturated level (86).
Two doses with equal toxicity were defined for each cell line, one considered as low dose, with at least 5% increased apoptosis in comparison with the non-irradiated controls, and the other as high dose’ chosen within the early plateau regions of the curves for each cell line (Table 1). The defined doses led to apoptosis rates within the range 10% - 20% for low doses and 40% - 60% for high ones (86).
| Fibroblast | Low DoseM J/m2 | High Dose, J/m2 |
|---|---|---|
| CS-B | 125 - 250 | 500 |
| XP-C | 375 - 500 | 750 - 1000 |
| FHN | 750 - 1000 | 2000 |
UVB Doses Defined as Low or High Based on the Apoptosis Levels (Sub-G1) Induce in Each Cell Line
2.3.2. Melanocyte
Melanocytes are specialized to produce melanin that is necessary for the pigmentation of some tissue such as skin, hair, eye, etc. A special organelle named melanosome is responsible to produce melanin from tyrosine or phenylalanine with the cooperation of some enzymes and proteins, and then the melanin is translocated to adjacent KC via dendrites. The key role of melanocyte is to protect skin from genotoxic stress of UV radiation via absorption role of melanin (87, 88).
Some anti-apoptotic factors are detected in melanocyte to regulate its functions such as those of Bcl2 family; MCL1, the homolog of Bcl2 is found in melanocytes; also, both c-IAP1 and c-IAP2 are strongly expressed in melanocytes. XIAP expression in melanocyte is low, but sufficient; livin is also expressed in melanocytes; though survivin- inhibitor protein of apoptosis (IAP) - FLIP, and caspase-8 inhibitor are not detected in normal melanocyte cells. On the other hand, there are some pro-apoptotic factors recognized in melanocyte such as the tumor suppressor p53 that are expressed weakly. Apaf-1, p53, dependent caspase-9 cofactor, and Bax (member of Bcl2 family) are eloquently expressed in cell lines of melanocytes (89).
2.3.2.1. Role of Melanocyte in UV Protection
UV radiation is a crucial factor that induces some dysfunctions such as loss of cellular integrity, DNA damage, and apoptosis in many types of skin cells; melanocyte via production of melanin protects skin against destructive effects by absorption of UV radiation (90). Responses of melanocyte to UV exposure is regulated by adjacent cells such as KC through secretion of paracrine factors such as α-MSH known as an essential factor with protective role against DNA damage (89). Nrf2 regulates secretion of α-MSH, responses of UVR mediated oxidative stress, and inflammatory responses of melanocyte to UVR; as well, it plays a protective role in UVB-induced CPD formation in melanocyte (91).
Response of melanocytes to UVR is via apoptosis process; by this way, expression of some apoptotic factors such as Mcl-1, XIAP, and livin are reduced. Also, expression of the tumor suppressor p53 is increased in this condition (89). In addition, phosphorylation and activation of p53 MAPKs, ERK, and JNK are the particular regulatory responses of UVR via apoptosis in melanocyte and KC (91).
2.3.2.2. Hair Melanocyte
Melanocyte stem cells are located in the hair follicle and play a pivotal role in the sustenance of periodical cycles of hair pigmentation. Melanocytes appear, proliferate, and differentiate into mature melanocytes in anagen phase of hair cycle. Apoptosis remove melanocytes from hair follicle in catagen phase and finally they are completely eliminated from hair cycle in telogen phase. When anti-apoptotic factors such as BCL2 are absent in hair follicle, no melanocyte stem cell exists due to apoptosis stimulation during the primary anagen. Selective loss of melanocyte stem cells during hair follicle morphogenesis is the result of the hair-graying phenotype of Bcl2 deficient. Another study indicates that removal of the stem cells is approximately related to ectopic differentiation of melanocytes (92).
2.3.3. Keratinocyte
The most specialized epithelial cells are KCs, which is the most sufficient cell type in the epidermis that synthesizes primary factors of barrier structure of skin by arranged differentiation. KC differentiation is enhanced by calcium, hydrocortisone, and vitamin A depletion and that differentiation is generally suppressed by retinoid and low calcium levels (93).
Apoptosis of KC presents a vital pattern in the modulation of proliferation and maintenance of epidermal thickness and removal of premalignant cells. Anti-apoptotic proteins such as Bcl2 family, its homolog, Mcl-1, and survivin are not detected in KCs; though Bcl-xl is distinguished. Also, both c-IAP1 and c-IAP2, XIAP, and livin are expressed weakly in KC lines. Moreover, the caspase inhibitor, FLIP, is highly expressed in KC. Pro-apoptotic factors such as the tumor suppressor, p53 and p53-dependent caspase-9 cofactor Apaf-1 are expressed weakly in KCs. Also, the Bcl2 family member, Bax, is absent from KC. Therefore, the anti-apoptotic protein FLIP is the factor expressed in highest amount in KCs, and Bcl2 family are the lowest expressed ones in this type of cells (89).
There are two important pathways of KC apoptosis. The first one is MAPK pathway that is activated via phosphorylation of epidermal growth factor receptor (EGFR) (94).
Subsequent phosphorylation and activation of MAPK kinases (MEKs) and extracellular signal-regulated kinases (ERKs) cause stimulation of a MAPK cascade via EGFR signaling. The second pathway occurs by involving phosphatidylinositol 3-kinase-mediated activation of the serine/threonine kinase Akt (95). Significant role of caspase-9 in KC apoptosis proved that Akt is a strong KC survival factor (96). Modulation of expression of IAPs or TRAF death receptor adapters activate NF-κB via Akt signaling may promote KC survival (97).
2.3.3.1. UVB-Induced Apoptosis in Keratinocyte
Apoptosis is the most protective factor against UVB-induced damage in epidermal KCs that rapidly eliminate premalignant cells. Activation of caspase-9 induces positive feedback modulation between caspase-3 and caspase-8 and plays the main role in UV-induced KC apoptosis independent of p53 (98).
Overexpression of anti-apoptotic proteins such as the IAP protein survivin and Bcl-2 can block the apoptotic responses of KCs (99). As a result, upregulation of anti-apoptotic factors such as Bcl-2 and IAP families can promote KC survival. Moreover, nerve growth factor and hepatocyte growth factor enhance levels of Bcl-2 and Bcl-xL and activate AKT to protect KC against UVB-induced damage (100).
2.3.4. Stem Cell
Stem cells are able to renew themselves and replace dead cells in the process of tissue repair. The cells that proposed to death, IAPs, are inactivated via antagonists. One of the antagonists is ARTS, a splice variant of the mammalian gene Septin4 (Sept4). ARTS is the only Sept4 isoform detected in HFSCs (hair follicle stem cell) that plays a pivotal role in wound healing and regeneration of hair follicles. The outer root sheath cells survive catagen in the absence of pro-apoptotic protein ARTS. At the end, these findings suggest that deficient of ARTS protects HFSCs against apoptosis. HFSC plays role in normal epidermis hemostasis, but in response to wound, it can enhance proliferation in epidermis and tissue repair, since Sept4/ARTS-/-HFSC are protected against apoptosis. In addition, XIAP has a crucial role in wound healing; therefore, it is a necessary factor for pro-apoptotic activity of ARTS. Conclusively, it was understood that apoptotic regulatory function of ARTS and XIAP can just be observed in hair follicle and not in more differentiated skin cells; but they attend the apoptotic pathways in HFSCs that can display significance therapeutic effects for wound healing and regeneration of hair follicles (101).
2.3.5. Hair Follicle
The HF is a cutaneous structure and has a specific cell cycle, which goes through courses of active hair growth (anagen), apoptosis progression (catagen), hair shedding (exogen), and relative resting (telogen) (102, 103). The activity of factors promoting the differentiation, proliferation, and survival predominates, cause HF growth; whereas, induction of apoptosis in HF occurs by activation of a type of signaling pathways, which cause HF regression (103, 104). Although catagen is often thought of as a regressive procedure, it is a wondrously organized energy-requiring remodeling process, whose progression assures restoration of further generation of HFs (105). Different cells in the HF show different behavioral patterns of apoptosis. The main follicular epithelial cells and melanocytes are the most susceptible cells to apoptosis. Though, fibroblasts, dermal papilla, and some of the melanocytes and KCs are resistant to apoptosis and are selected for survival (104); macrophages phagocyte the apoptotic cells in the HF and neighboring epithelial cells fill the gap left by apoptotic cells (103). In the hairless gene mutation, the ability of the HF to reenter anagen is lost due to the dermal papilla and the HF epithelium collapse during catagen (106).
Withdrawal of growth factors that help differentiation and proliferation of the cells in the anagen phase and signaling by death receptors prompt the physiological ability of the HF. According to evidence, apoptosis regulation of varied distinct of HF is occurred in different pathways (107) and faults in the apoptosis regulation can cause many problems, similar to hair growth disorders (hirsutism and various hair loss conditions) (102). Also, mutation in apoptosis pathways in HFs plays the main role in hair loss disorders such as: chemotherapy-induced hair loss and androgenetic alopecia (108). Based on these findings, novel drugs for apoptosis regulation (for example, synthetic p53 and caspase inhibitors, TNF-α-inhibiting antibodies, Bcl-2/BclxL antisense oligonucleotides) are recognized (109). Besides, decreasing apoptosis activity in the HFs and suppression of TGF-β can be effective in the treatment of alopecia disorder (male pattern baldness) (107).
2.3.6. Dermal Papilla Cell
The dermal papilla is a dense association of specialized dermis-derived stromal cells positioned at the base of the hair follicle, which lengthens the hair growth phase by signaling the follicular epithelial cells (110).
The aggregated human dermal papilla cells express the hematopoietic stem/progenitor cells (HSPC016)-related gene (111).
The biological activities of the dermal papilla cells such as aggregative behavior and hair cycle regulation might have contribution with the HSPC016 expression. But the definite roles of the gene HSPC016 in follicle development and in hair cycling need to be proved by more studies. It is interesting to observe that the death of both the dermal papilla cells and the 3T3 fibroblast result in HSPC016 expression (110).
3. Conclusions
The role of apoptosis in treating diseases is through various factors and pathways; regulation of p53 function is very important to regulate many actions and cellular responses. Also, the role of this factor in regulating signals associated with KC, and melanocyte is quite vital.
UVB causes an increase in the factor p53; therefore, increases the induction of apoptosis in skin cells. Also, p53 and p21 (alternative of p53) play a significant role in signaling of aging.
Regulating the expression of BCL2 and FasL proteins can play an important role in the induction of apoptosis for treatment aspects. For example, expression of FasL in TEN, EM, SLE, pemiphagus, DH, and eczematous are increased and the expression of BCL2 in SLE, and pemiphagus are decreased. Therefore, by regulating the expression of these proteins, it is possible to achieve therapeutic purposes.
Bcl2 family proteins such as MC1 and c-IAP1, c-IAP2, XIAP, and livin are expressed sufficiently in melanocyte in comparison with other apoptotic proteins such as survivin, FLIP, and caspase 8 inhibitor. Also, NRF2 regulates melanocyte responses against UV-exposure with α-MSH expression.
FLIP is the most expressed and BCL2 family the least expressed factors in KC, though Bax is not expressed in KC at all. There are two pathways in KC apoptosis, the first one is MAPK that phosphorylation of EGFR plays a pivotal role in activation of this pathway. The second pathway is AKT (independent of p53). Caspase 9 is the most key factor in this pathway and provides a positive feedback between caspases 8 and 3. Also, some growth factors increase the AKT activity and protect KC from apoptosis.
ARTS is the antagonist of anti-apoptotic proteins. Therefore, in the absence of this gene, HFSCs are protected against apoptosis. Also, XIAP have crucial effect on ARTS regulation for wound healing process.
The cells with the highest sensitivity to apoptosis are melanocyte and follicular epithelial cell though dermal papilla cell, fibroblasts, and some of KCs are the cells with the highest resistance to apoptosis.
According to the current review study, novel drugs can be designed based on stimulation or inhibition of the apoptosis such as BCL2 inhibitors, p53 inhibitors, and caspase inhibitor.