1. Background
Free radicals and their role in the incidence of various diseases have attracted the attention of many researchers (1). The animal body naturally responds to destructive effects of incoming free radicals in various ways. One of these methods is the use of antioxidants to neutralize the degrading nature of free radicals. The disruption of balance between the production of free radicals and antioxidant mechanisms leads to destructive conditions in the body known as oxidative stress (2). Previous studies have shown that oxidative stress can severely damage various body biomolecules, including carbohydrates, lipids, proteins, and DNA of living cells (3). Up to the present, various reasons have been reported to be causes behind increased oxidative stress in the human body. Stressful working conditions, smoking, inadequate nutrition and physical activity and training (T) are among the causes of increased oxidative stress in the blood. For example, it has been shown that the oxidative stress level in the blood of firefighters, military personnel and police officers is above normal as a result of their working conditions and therefore they are more prone to cardiovascular diseases (4). Apoptosis is a physiological process in the body to remove old and worn-out cells or cells whose DNA is irreparably damaged (5). Signals of apoptosis initiation in the cell include activation of a group of cysteine hydrolyzing enzymes known as caspases. Apart from apoptosis, caspases are a group of proteases that are involved in many other cell processes, such as cell differentiation (6). One sign of increased oxidative stress in living cells is an increase in apoptosis-related signals such as caspase-3 and release of cytochrome-C from mitochondria in stressed cells (7). Release of cytochrome-C from mitochondria activates caspase-9, resulting in caspase-3 activation and eventually apoptosis. Thus, increased oxidative stress indirectly induces apoptosis in healthy cells (8). Despite numerous benefits of exercise in the prevention of neurological and cardiovascular diseases, it has been found that oxidative stress increases in the body after exercise (9). Mitochondrial activity, when using muscles to supply the required energy, is the major cause of increased oxidative stress during exercise (10). It should be noted, however, that the amount of oxidative stress produced is directly proportional to the intensity of pressure applied to the muscles and the duration of exercise (11). In this vein, adding different antioxidants to the diet is recommended by many researchers for the maintenance of balance between free radicals and antioxidants and also due to the ability of cells to counter these dangerous compounds (12). Given obstacles and difficulties associated with existing therapeutic and control methods, the need for new and effective treatments is quite evident. Coriander, known as Coriandrum sativum, belongs to the Apiaceae family (13). The therapeutic potential of coriander seed (CS) extracts has been investigated in different studies. CS has been shown to have antimicrobial (14), anti-cancer (15) and rich antioxidant properties (16). These therapeutic properties of CS are thought to be related to essential fatty acids extracted from different parts of the plant (17). Previous studies have also shown that the extract of CS has a significant effect on the reduction of oxidative stress in the brain and testis of rats (18).
In previous studies, effects of T and CS consumption have been separately examined on apoptosis-related factors and various results have been reported. However, no study has been found to investigate the simultaneous effects of T and CS. Thus, the present study aimed to investigate the effects of T with CS consumption on caspase-3 and cytochrome-C in the heart tissue of hydrogen peroxide (H2O2) - poisoned rats.
2. Methods
In this experimental study, according to previous studies (18, 19), 35 male Wistar rats with the mean age of 10 - 12 weeks, weighing 200 - 220 g, were purchased and transferred to the laboratory. After one week of laboratory adaptation, the rats were divided into 7 groups of 5 rats including: (1) healthy control, (2) sham, (3) T, (4) 500 mg/kg CS, (5) 1000 mg/kg CS, (6) T+500 mg/kg CS, and (7) T+1000 mg/kg CS. During eight weeks, the groups 2 - 7 received 1 mmol/kg H2O2 (Sigma Aldrich) peritoneally three times per week (19) and the groups 4 - 7 received CS extracts at given doses (20). Moreover, the groups 3, 6, and 7 performed T three days per week (21).
For preparation of the CS extract, 400 g CS powder was macerated with 2160 mL of EtOH-H20 (80:20) for 24 hours. The extract was then shaken, filtered and evaporated in a rotary evaporator under reduced pressure until a semisolid extract was obtained. Moreover, the concentrated extract was freeze-dried to obtain a dry powdered extract with a yield value of 10/85% (W/W) (20).
To perform T, the rats ran on a treadmill at a speed of 8 m/min and slope of 10° for 30 minutes in the first week, at a speed of 12 m/min with the same slope for 30 minutes in the second week, at a speed of 16 m/min with the same slope for 45 minutes in the third week, and at a speed of 20 m/min with the same slope for 45 minutes in the fourth week. During the fifth to eighth weeks, the rats were trained at a speed of 20 m/min and slope of 10° for 60 minutes each session (21). Forty eight hours after the last T session and CS extract consumption, to collect heart tissue specimens, the rats were anesthetized with ketamine 10% (50 mg/kg dose) and xylazine 2% (10 mg/kg dose) and heart tissues were extracted. After weighing and washing the extracted heart tissues, they were frozen in liquid nitrogen and stored at -80° C for subsequent measurement. The tissues were then incubated for evaluation of the research variables. After homogenization of the solution, it was centrifuged at 9000 rpm for 10 minutes at 4°C and supernatants were separated.
Caspase-3 and cytochrome-C were measured by ELISA using CUSABIO laboratory kits with catalog numbers CSB-E08857r and CSB-E14281r, respectively.
2.1. Statistical Analysis
Independent-sample t-test was used to evaluate the effects of H2O2 poisoning on caspase-3 and cytochrome-C between the healthy control and sham groups. Furthermore, two-way ANOVA with Bonferroni’s post hoc tests were used to review the effects of T and CS on caspase-3 and cytochrome-C by SPSS (version 21) software (P ≤ 0.05).
3. Results
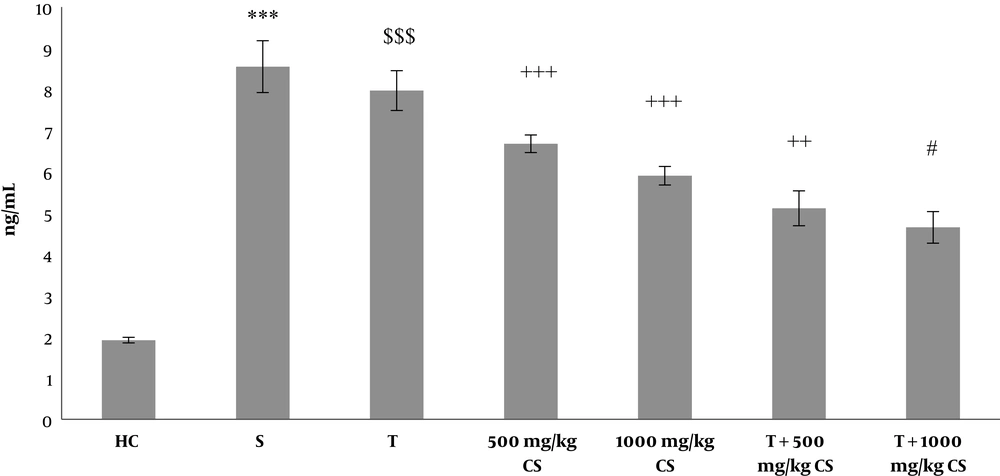
Levels of caspase-3 and cytochrome-C in the heart tissues of the rats in the seven research groups are presented in Figures 1 and 2, respectively. The results of the Independent-sample t-test showed that caspase-3 and cytochrome-C levels were significantly higher in the sham group than in the healthy control group (P = 0.001) (Figures 1 and 2). According to the two-way ANOVA results, T (P = 0.001) as well as 500 mg/kg and 1000 mg/kg CS (P = 0.001) significantly decreased caspase-3 compared to the sham group. In addition, interactive effects of T and CS were significant on caspase-3 decrease compared to the sham group (P = 0.04). Bonferroni’s post hoc test results revealed that 500 mg/kg and 1000 mg/kg CS consumption had a significant effect on decrease of caspase-3 compared to the sham group (P = 0.001), with 1000 mg/kg CS having a greater effect in this regard compared to 500 mg/kg CS (P = 0.009) (Figure 1).
Caspase-3 levels in the heart tissues of the seven groups of rats. Data are presented as mean ± SEM. Statistical analyses were performed using two-way ANOVA with Bonferroni’s post hoc tests. ***, P < 0.001 comparison of the sham group with the healthy control group; $$$, P < 0.001 comparison of the training group with the sham group; ++, P < 0.01 and +++, P < 0.001 comparison of the treatment group with the sham group; #, P < 0.05 comparison of T+500 mg/kg CS with T+1000 mg/kg CS. CS, coriander seed, HC, healthy control, S, sham, T, training.
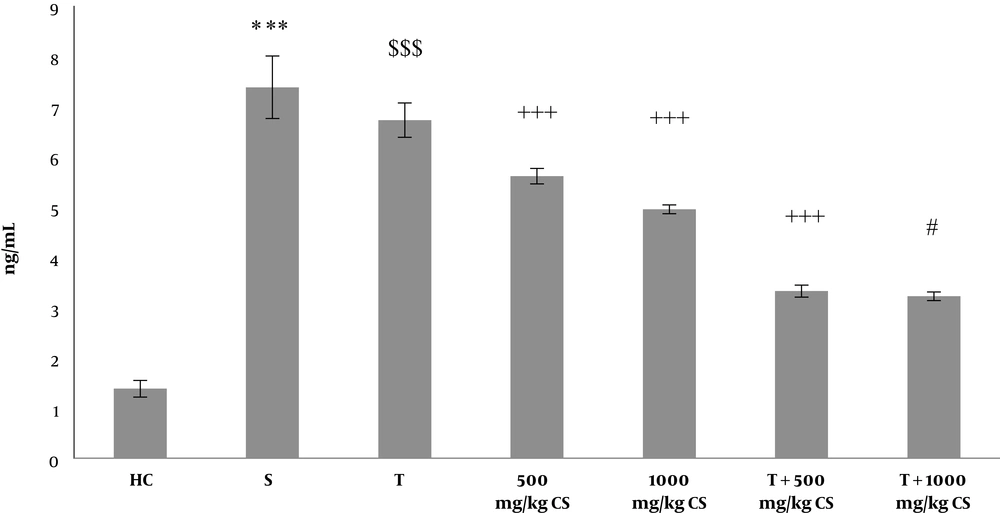
The results of two-way ANOVA also indicated that T (P = 0.001) along with 500 mg/kg and 1000 mg/kg CS (P = 0.001) significantly decreased cytochrome-C compared to the sham group. Further, interactive effects of T and CS were significant on decrease of cytochrome-C compared to the sham group (P = 0.001). Based on the Bonferroni’s post hoc test results, consumption of 1000 mg/kg and 500 mg/kg CS had a significant effect on decrease of cytochrome-C compared to the sham group (P = 0.001), with 1000 mg/kg CS having a higher effect in this regard compared to 500 mg/kg CS (P = 0.001) (Figure 2).
Cytochrome-C levels in the heart tissues of the seven groups of rats. Data are presented as mean ± SEM. Statistical analyses were carried out using two-way ANOVA with Bonferroni’s post hoc tests. ***, P < 0.001 comparison of the sham group with the healthy control group; $$$, P < 0.001 comparison of the training group with the sham group; +++, P < 0.001 comparison of the treatment group with the sham group; #, P < 0.05 comparison of T+500 mg/kg CS with T+1000 mg/kg CS. CS, coriander seed; HC, healthy control; S, sham, T, training.
4. Discussion
In the present study, H2O2 poisoning significantly increased caspase-3 and cytochrome-C in the heart tissues of the rats. In fact, H2O2 in the study was used as an oxidative stress enhancer. The results showed that the gene expression levels of caspase-3 and cytochrome-C significantly increased in the heart tissues of rats receiving H2O2 compared to the healthy control rats. As a result, the heart tissues of rats exposed to H2O2 were destroyed. Previous studies have also shown an increase in oxidative stress after exposure of human cells to H2O2 (22). Consistent with the present study, Pirooz et al. showed that eight weeks of H2O2 administration at doses of 1 and 2 mmol/kg led to a significant increase in apoptotic factors (23). Several mechanisms have been implicated in the induction of apoptosis in the myocardial tissue of the heart, including the role of oxidative stress. Oxygen free radicals resulting from oxidative stress along with inactivation of ERK1-2 kinase and inactivation of another kinase enzyme called C-juN/C-juN/AP-1 may indicate apoptosis following oxidative stress (23).
In this study, eight weeks of T resulted in a significant decrease in caspase-3 and cytochrome-C in the heart tissues of rats poisoned with H2O2. Consistent with the findings of the present study, it was observed that low-intensity endurance training could affect oxidative stress factors and that oxidative stress would be significantly reduced if T continued for at least 12 weeks (24). Eight weeks of continuous endurance training resulted in improved citrate oxidase activity and oxidative stress in the rats (25). Exercise activity appears to be directly hemoligomerized by increasing levels of Bcl-2 that acts directly on the mitochondrial outer membrane and plays a critical role in mitochondrial outer membrane permeability. Moreover, increased Bcl-2 following T adaptation inhibits the Bax/Bcl-2 ratio. This, subsequently, alters the mitochondrial membrane potential and release of cytochrome-C and Smac/DIABLO, which eventually inhibits caspase-9. However, the major role of mitochondria in inhibiting apoptosis following T is associated with inhibition of cytochrome-C release into cytosol, which leads to increased mitochondrial membrane potential. This potential is essential for the production of ATP and the maintenance of cellular homeostasis. Finally, a decrease in cytochrome-C inhibits apoptosis by decreasing caspase-9 production and induces proteolytic inhibition of effector caspases (26). In addition to exercise activities, the role of medicinal plants in reducing oxidative stress has been investigated in various studies (13, 15, 27, 28).
The results of the present study showed that eight weeks of CS consumption significantly decreased caspase-3 and cytochrome-C in the heart tissues of rats poisoned with H2O2. In addition, consumption of 1000 mg/kg CS had a greater effect on the decrease of caspase-3 and cytochrome-C compared to 500 mg/kg CS consumption. These findings suggest dose-dependent effects of CS, which is known as a medicinal plant with many properties. Consistent with the findings of the present study, the heated extract of coriander leaves significantly reduced the amount of oxidative stress-related factors in the kidney tissue of mice (27). CSs have also been reported to be rich in phenolic compounds with antioxidant properties and CS consumption can be effective in reducing the levels of oxidative stress (28). Various studies have reported that anti-toxic effects of coriander can be due to its phenolic compounds and antioxidants (29). Phenol contained in coriander can reduce H2O2 production and prevent oxidative stress (29).
The results of our study also demonstrated that T along with CS consumption had an interactive effect on decrease of caspase-3 and cytochrome-C in the heart tissues of rats poisoned with H2O2. In this regard, T and 1000 mg/kg CS consumption together had a greater effect on the decrease of caspase-3 and cytochrome-C compared to simultaneous T and 500 mg/kg CS consumption. Lack of sufficient information on the effect of T and CS consumption on caspase-3 and cytochrome-C in the heart tissues in H2O2 toxicity status and lack of access to cell death assay methods appear to be limitations of the present research. Therefore, it is suggested in future studies to investigate the effect of T with different intensities along with CS consumption on apoptosis indices and also to use hematoxylin-eosin (H&E) and tunnel techniques.
4.1. Conclusions
Considering the results of the present study, it appears that in the context of H2O2 poisoning, caspase-3 and cytochrome-C increase in heart tissues of rats. However, T and CS consumption, both together and alone, can prevent an increase incaspase-3 and cytochrome-C in heart tissues of rats poisoned with H2O2. Moreover, effects on CS consumption can be dose-dependent.