1. Background
The thyroid is an endocrine gland that plays an important role in metabolic processes. Although this gland is very small, it has important duties, including regulation of cell metabolism, controlling growth and development, regulating reproductive performance, and so on. Triiodothyronine (T3) and thyroxine (T4) are important thyroid hormones that play a role in regulating the metabolic rate of target tissues such as the liver, heart, brain, and kidneys (1, 2). Thyroid functional disorders are relatively common in the general population (3-5). Disruption of thyroid function can lead to hypothyroidism or hyperthyroidism. The incidence of these disorders in women is about 5 to 10 times higher than in men. Also, these diseases are associated with an elevated risk of digestive, subjective and metabolic disorders, heart diseases, and infertility in men and women (6-8)
Oxidative stress is the condition in which active species of oxygen, nitrogen, and free radicals are produced in excess from the metabolic processes within the cell and can cause cell toxicity (9). The oxidative damage caused by these species contributes to the pathogenesis of many endocrine gland diseases, such as thyroid disorders. Thyroid hormones play an important role in the oxidation state. Therefore, their regulatory role in the production of free radicals is notable. On the other hand, these hormones are effective in the synthesis of antioxidant enzymes, vitamins, and regulatory proteins. The production of thyroid hormones is an oxidative biochemical reaction that depends on the formation of peroxides. The level of thyroid hormones, due to their effect on mitochondrial respiration, can be a physiological regulator of oxidative stress in cells (10-12).
According to the literature, thyroid deficiency has been shown to reduce free radicals due to a reduction in thyroid hormone levels (13). The effect of hypothyroidism on antioxidant enzymes has been studied in several studies, but they have yielded contradictory results. Also, in hyperthyroidism, increased metabolic rate, elevated levels of free radicals, and increased (14) or decreased (15) antioxidant enzymes have been shown. Moreover, the available data concerning oxidative stress in both hypothyroidism and hyperthyroidism are scarce and controversial, and most of these studies have been performed on rat tissues.
2. Objectives
In this study, we evaluated the oxidation state and the levels of some biochemical parameters in the serum of hypo and hyperthyroid rat models and compared them with each other.
3. Methods
3.1. Animals
In this experimental study, adult male Wistar rats were kept at the Center of Experimental Medicine of Birjand University of Medical Sciences. These rats weighed approximately 200 ± 10g at the beginning of the study. They were held under controlled temperature and lighting conditions (12 hours light/12 hours dark, 21 ± 2°C). The rats had free access to drinking water and food. They were divided randomly into three groups of seven: one group was treated with propylthiouracil as the hypothyroid group, another group was treated with levothyroxine as the hyperthyroid group and the final group as a control.
3.2. Treatment
To induce hyperthyroidism in the rats, we used two LTX (levothyroxine) tablets (50 µg- Iran hormone) in 100cc drinking water. For the induction of hypothyroidism, a PTU (propylthiouracil) tablet (50 mg-Iran hormone) was used per 100cc of drinking water and administered to the intervention groups. The tablets were ground separately in a mortar and dissolved in rats' drinking water bottles, which were used daily. This procedure was repeated every 24 hours for five weeks (16, 17). All three groups were kept in one place during the intervention period and had the same amount of water and food, and the water bottles of each group were changed daily. All rats were weighed before and after the research protocol by means of a digital scale (Sartorius, Germany)
3.3. Sample Collection
After measuring the final weight of the rats, they were anesthetized with ether, blood samples from the heart of the rats were collected using a piston syringe, and poured into anticoagulant tubes, then centrifuged at 2000 rpm for 10 minutes. Serum samples were separated and stored at -20°C for analysis.
Measurement of T3 and T4 and biochemical parameters:
In order to ensure the induction of hypothyroidism and hyperthyroidism in rats, T3 and T4 levels were measured by the chemiluminescence method using DiaSorin kits. Serum uric acid (UA), lactate dehydrogenase (LDH), alkaline phosphatase (ALK), aspartate aminotransferase (SGOT or AST) and alanine aminotransferase (SGPT or ALT), LDL (low-density lipoprotein), HDL (high-density lipoprotein), triglyceride (TG), and total cholesterol (TC) levels were measured by an automated analyzer using standard kits (Pars Azmoun, Iran).
3.4. Measurement of Total Antioxidant Capacity
Serum total antioxidant capacity (TAC) was measured by the ferric reducing antioxidant power (FRAP) method with the modified microplate technique. This method is based on the ability of the plasma to reduce ferric ions (Fe3+) to ferro (Fe2+) in the presence of Tripyridyl-S-triazine (TPTZ), which is used as a reagent. In this reaction, Fe+2 and TPTZ create a blue-colored complex with a peak absorption of 593nm. The intensity of the produced color is directly linked to the potency of the antioxidants (18).
3.5. Measurement of Malondialdehyde
Malondialdehyde (MDA) level is used as an index of lipid peroxidation. It reacts with thiobarbituric acid (TBA) as a thiobarbituric acid reactive substance (TBARS) and produces a purple-colored complex. The adsorption intensity of this complex is determined by a spectrofluorometer at 515 nm as the excitation wavelength and 553 nm as the emission wavelength (19).
3.6. Measurement of Total Thiol Group
Total thiol groups were measured by the Ellman method. The substance 5, 5’-dithiobis-2-nitrobenzoate (DTNB) is used as the reagent to measure total thiol concentration. In addition, DTNB reacts with thiol groups and produces a yellow-colored complex with a peak absorbance at 412 nm (20).
3.7. Statistical Analysis
The results were analyzed using SPSS software, version 16. The data were expressed as mean ± SD (standard deviation). Student’s t-test and Mann-Whitney U test was used for normally and abnormally distributed data, respectively. A one-way analysis of variance (ANOVA) test was carried out to differentiate between the groups. In all statistical tests, a P value of less than 0.05 was considered significant.
4. Results
This study was carried out to compare the biochemical and oxidative stress parameters among the three groups of control, hypothyroid, and hyperthyroid rats. The changes in the mean initial weights of the rats compared to their final weights were significant in the control and hyperthyroid groups, but this change was not observed in the hypothyroid group based on Student’s t-test (Table 1).
The mean levels of T3 and T4 hormones in the hypothyroid group were lower than those in other groups, and in the hyperthyroid group, it was significantly higher than the others based on the ANOVA test (Table 2).
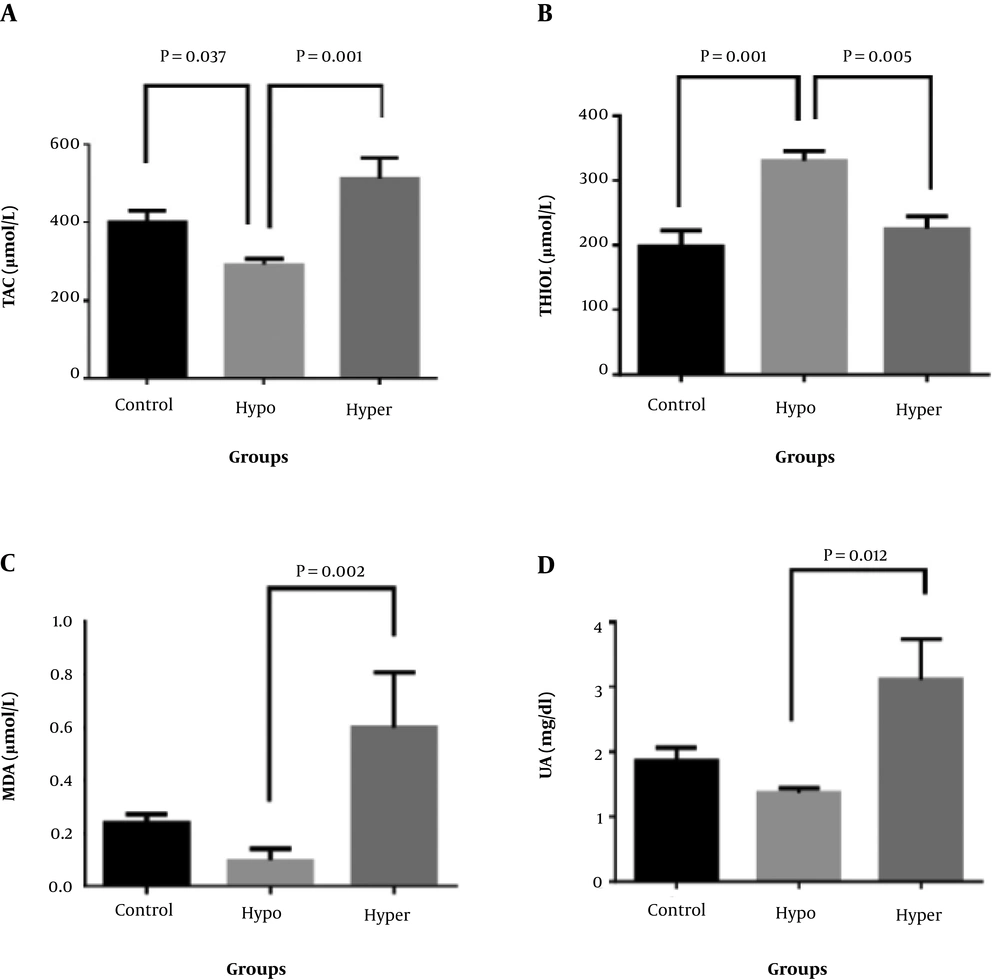
There was also a significant difference in the mean levels of TAC, thiol, MDA, and UA in the three groups (P < 0.05). The mean levels of TAC, MDA, and UA in the hyperthyroid group were significantly higher than the hypothyroid group, but the mean of thiol in the hypothyroid group was significantly higher than that of the hyperthyroid group. In addition, the mean of the TAC level in the hypothyroid group was significantly lower than the control group (P < 0.05) and the mean of the thiol group in the hypothyroid group was significantly higher than the control group (P < 0.05), but there was no other significant difference between the groups compared to the control group (Figure 1).
Comparison of oxidative stress parameters and UA in hypothyroid (Hypo) and hyperthyroid (hyper) groups compared to the control group. (A) TAC (total antioxidant capacity) levels; (B) Thiol group concentration; (C) MDA (malondialdehyde) levels; (D) UA (uric acid) concentrations. Values are given as mean ± standard deviation (SD) of seven animals. P < 0.05, considered as significant data.
In terms of biochemical parameters among the groups, the mean of LDH in the hyperthyroid group was higher than the control group. It also showed a significant difference in comparison to the hypothyroid group, but there were no significant differences in the means of the other biochemical parameters based on the ANOVA test (Table 3).
| Control | Hypothyroid | Hyperthyroid | P Value | |
|---|---|---|---|---|
| LDH, U/L | 857.2 ± 427.9 | 701.2 ± 294.2 | 1370 ± 465.0 | 0.01b |
| ALP, U/L | 766.5 ± 261.0 | 1338.0 ± 572.0 | 1126.2 ± 979.8 | 0.2 |
| SGPT, U/L | 78.8 ± 15.1 | 130.4 ± 106.9 | 102.5 ± 33.9 | 0.3 |
| SGOT, U/L | 147.0 ± 24.0 | 189.0 ± 84.5 | 195.7 ± 54.5 | 0.2 |
| LDL, mg/dL | 15.7 ± 3.8 | 17.1 ± 3.8 | 15.4 ± 2.5 | 0.6 |
| HDL, mg/dL | 53.1 ± 4.6 | 52.1 ± 2.9 | 53.4 ± 11.5 | 0.9 |
| TG, mg/dL | 43.4 ± 28.4 | 29.5 ± 15.8 | 44.1 ± 28.3 | 0.4 |
| TC, mg/dL | 74.0 ± 9.7 | 87.2 ± 14.3 | 78.0 ± 14.5 | 0.1 |
Comparison of Biochemical Factors in Different Experimental Groupsa
5. Discussion
Thyroid hormones play an essential role in the regulation of metabolic processes and oxidative stress. In this study, the reduction of T3 and T4 levels in the hypothyroid group and the increased levels of these hormones in the hyperthyroid group compared to the control group indicated the effect of PTU and LTX on the target rat groups (21). The rising of rats' weight has happened during the natural growth process, and there was a significant difference in the mean weight between the control and hyperthyroid groups before and after the study, but this difference was not observed in the hypothyroid group; the mean weight of this group was approximately the same before and after the study, which could be due to reduced food consumption and the effect of PTU, confirming the induction of hypothyroidism in this group. Das and Chainy also showed that the mean weight of PTU-treated rats was reduced relative to the final weight of rats in the control group (22). These results are also consistent with the findings of Tas et al., who showed that the mean weight of the group treated with PTU was lower than that of the control group (23). Although It seems that the consumption of levothyroxine in the hyperthyroid group may increase the amount of consumed food, and despite the increase in the rate of metabolism, the weight of rats has increased like the control group.
While the mean weight of the control group was significantly higher after the observation period due to normal growth and being older, this difference was not seen in hypothyroid groups, possibly because of the effect of PTU, which reduces the appetite, so they did not gain weight significantly. Consistent with our result, Das and Chainy also showed that the mean weight of PTU-treated rats was reduced relative to the final weight of rats in the control group (22). These results are also consistent with the findings of Tas et al., who showed that the mean weight of the group treated with PTU was lower than that of the control group (23).
Interestingly in the hyperthyroid group, the mean weight was increased significantly after the intervention period, similar to the control group. Although levothyroxine increases the rate of metabolism, it may also increase food intake in animals, and so this could be a reason for gaining weight in the hyperthyroid group (24).
In relation to oxidative stress parameters, the mean of TAC in the hyperthyroid group was significantly higher than that in the hypothyroid group, and in the hypothyroid group was significantly lower than the control group. According to previous studies, it can be argued that T3 and T4 thyroid hormones in humans and animals play an important role in maintaining the oxidative stress balance and the fight against oxidative stress, such that the reduction of these hormones in hypothyroidism reduces the rate of metabolism and thus reduces the probability of free radical production, thereby reducing TAC. On the other hand, an increase in these hormones in hyperthyroidism promotes the rate of metabolism, enhancing the probability of elevated production of free radicals and increased level of antioxidants in order to neutralize the effects of free radicals. Also in a study by Saleh, the TAC level in the hypothyroid group was lower than that in the control group. Bhimte et al. also showed that as serum TSH (thyroid-stimulating hormone) levels increase, TAC levels diminish, because TSH may increase the secretion of inflammatory cytokines and lower antioxidant levels (25).
The UA in the hyperthyroid group was significantly higher than that of the hypothyroid group, which can be explained by the increased levels of thyroid hormones that are associated with an enhanced metabolic rate. This is in line with the results of the study by Sato showing that UA in patients with hypothyroidism significantly decreases but increases in patients with Graves’ disease (26). However, it is contradictory to the results of Liang, since he reported a significant increase in UA in subclinical hypothyroid patients compared to a control group (27). In the mentioned study, TAC was measured by the FRAP method (18). In this method, the major part of serum or plasma antioxidant capacity is related to the amount of uric acid in these specimens, and the elevated level of uric acid in hyperthyroid rats will also increase TAC.
The average MDA index in the hyperthyroid group was significantly higher than that of the hypothyroid group, which can be due to the increased rate of metabolism in hyperthyroidism relative to hypothyroidism and as a result of increased lipid peroxidation rate of the active group. As Petrulea in his study showed, the MDA level decreased in the hypothyroid group compared to the control group (28). In spite of these results, in the study by Das and Chainy, after the induction of hypothyroidism, the level of lipid peroxidation did not change, while after treatment with T3, it increased (22). Also, in the study by Erdamar, the levels of MDA increased in the hypo and hyperthyroid groups, while it diminished in the hyperthyroid group after PTU treatment (13).
The mean level of thiol in the hypothyroid group was significantly higher than that of the hyperthyroid group, which can be due to the fact that in hypothyroidism due to a lower metabolic rate than hyperthyroidism, the oxidation of thiol groups in proteins and the formation of disulfide bonds happen less frequently. However, Ates et al. in a study of subclinical hypothyroid patients reported a reduction in thiol groups and increased di-sulfide bonds (29), resulting in an increase in the serum levels of thiol groups. In the study of Komosinka, which was performed on patients with Graves’ disease (a type of hyperthyroidism), thiol levels decreased in patients due to increased oxidative stress and free radical production, followed by increased disulfide bonds and declined thiol levels in these patients (30).
Regarding biochemical factors such as LDH, there was a significant increase in the level of this enzyme in the hyperthyroid group compared to the hypothyroid group, which is in agreement with the study of Messara (31). The increased level of this enzyme in the hyperthyroid group can be due to the elevated metabolic rate and oxidative stress in hyperthyroidism and increased blood acidity. There was no significant difference in the other biochemical parameters among the studied groups, although, in the Oktay study, the mean levels of TC, TG, LDL-c, and HDL-c in the hyperthyroid rats were significantly lower than those in the control group, and in the hypothyroid group, it was significantly higher than the control group (32). In the study by Saleh, the means of LDL and cholesterol levels in the hyperthyroid group were significantly higher than those in the control group (21). These differences can be related to the method of hypothyroidism and hyperthyroidism induction, the length of the treatment course and the drug dosage.
5.1. Conclusion
Based on the results obtained in this study, hypothyroidism and hyperthyroidism are associated with the deterioration of the oxidation state and an impairment of the antioxidant defense system, and the use of various supplements and antioxidants to improve this condition can be helpful. Although hyperthyroidism has been observed to increase total antioxidant capacity, it seems to be due to an increase in some endogenic antioxidants such as uric acid due to enhanced metabolism and free production radicals. On the other hand, the decrease in total antioxidant capacity and the increase in thiol groups in the hypothyroid group indicates an imbalance in the antioxidant defense system.