1. Background
The first identified pandemic respiratory involvement due to corona viruses, which is known as severe acute respiratory syndrome (SARS), had been firstly reported from Guangdongin Southern China in 2002 (1) and the next pandemic was the Middle East respiratory syndrome (MERS), which had been firstly reported from Saudi Arabia in 2012 (2). These diseases had been reported to have considerable morbidity and mortality rates (3). The third pandemic coronavirus, which is novel coronavirus disease-19 (COVID-19), had been identified to be caused by a RNA virus, called SARS coronavirus 2 (SARS-CoV-2), which had been originated firstly from China (4). SARS infection may result in to higher leukocyte numbers adding to respiratory disorders, and valuable increase in pro-inflammatory cytokines in blood plasma had been reported from COVID-19 infected patients (5).
Several public and individual strategies against pandemic diseases had been introduced and are employed now. Among them adequate nutrition and immunonutrition had been reported as an important preventive factor, which had been known as “prehabilitation”. Prehabilitation can also help the body to act potently against the lethal pathogens like COVID-19 (6, 7). Prehabilitation is defined as an important intervention against any kind of stress in the way that patient’s health becomes supported and makes him/her to feel better (6), and they will then better fight with stress and make it defeated (7, 8). Efficient nutrition as important factor for the immune system activation, play the unique role in clearance of the body from viral infections and become immediately healed after severe viral infections (8).
In order to be in health and prevention of different infections including viral infection like COVID-19, permanent efficiency and awareness of immune system against any kind of risk and/or pathogen’s invasion is required adding to its ability in differentiation between pathogens and self and/or immerse or useful molecules for body (8, 9). Macronutrients, micronutrients, and the gut microbiome had been reported to have important roles in efficient immune reactions against pathogens (7, 8, 10). Direct, indirect actions of nutrition on immune system, which may result in changing of immune cells functions and changing of normal microbial flora of the gastrointestinal tract consequent to nutrition, are common known effects of nutrition on immune system (8, 11).
There are several reports about effect of nutrition on immune system (9-12), and vitamins A, D, C, E, B6, B12, folate, copper, iron, zinc, and selenium had been introduced to have potential synergistic relationships in augmentation of immune system, and among them role of vitamin C, D, and zinc had been strongly approved and they had been reported as important factors on immune system potency (9). In this review role of different vitamins on intensification of immune system and their probable role on prevention/treatment of COVID-19 infection have been reviewed. Dominant hypothesis of current study is to enhance this fact that nutrition can act as an important factor in prevention of infection including COVID-19.
2. Method
Current systematic review had been designed based on this main hypothesis that vitamins, as important immune-modulator micronutrients, may have important roles in prevention and/or treatment of severe viral infection including COVID-19 infection. In this way, during the interval of first epidemic of COVID-19 (December 2019) till April 2020,totally 120 related articles based on the chosen keywords including: COVID-19, enhancement, immune system, modulation, prevention, treatment, viral infection and vitamins via using and/plus method, had been extracted from common indexing databases/websites including: Google Scholar, Pub Med, Scopus, and other indexing websites. Among mentioned articles 63 articles had been omitted for this study due to their unrelated subjects or their repeat. Finally 57 evaluable highly approved and related published articles, in the interval time of January 1973-April 2020, had been selected for this study. Their topics had been classified to different main vitamin groups and subgroups for vitamin B family according to their studied vitamins. Full text of mentioned articles had been purchased and/or downloaded and then studied carefully, also available data about role of vitamins on prevention and/or treatment of COVID-19 infection had been reviewed, and finally their contents had been purified and listed in different categorizations (Table 1). Differential percentages of studies among whole of reviewed studies, differential percentages of studies about association with COVID-19 and mechanisms of vitamins effects on immune system for every vitamin had been reviewed. To the best of author’s knowledge and date of design and searching, there were no recent systematic reviews on effects of vitamins on prevention/treatment of COVID-19 infection and this is the first study which discusses about role of vitamins on prevention/treatment of COVID-19 infection.
| Reference Number | Category | Focused on Vitamin/s | Dose | Route | Duration of Administration, d | Main Findings |
|---|---|---|---|---|---|---|
| (13) | Review | A, Bs, C, D, E | - | - | - | Efficient integrity of the mucosal epithelium |
| (14) | Review | A, D | - | - | - | Enhance the immune system |
| (15) | Review | A | - | - | - | Modulate immune system |
| (16) | Review | A | - | - | - | Modulator of the immune system via enhance immune cells proliferation |
| (17) | Animal study | A | 25 - 300, 1000 μg/mouse/day | Injection | 7 | T killer cell induction |
| (18) | In-vitro | A | - | - | - | Stimulates proliferation of T- Cells |
| (19) | Animal study | A | - | - | - | Stimulates proliferation of T-cells |
| (20) | Human study | A | 100.000 IU | Orally | - | Stimulation of immune system |
| (21) | Human study | A | 100.000 IU | Orally | - | Stimulation of immune system |
| (22) | Animal study | A | 400 IU, 0.1 mL | Injection | 7 | Reduce immune suppressive action of cortisone |
| (23) | Review | A | - | - | - | Modulator of the immune system |
| (24) | Animal study | B1 | - | - | - | Enhance lymphocytes activities |
| (25) | Review | B complex | - | - | - | Stimulation of the immune system functions |
| (26) | Animal study | B2 | 10 mg/kg bw/6 h, 80 mg/kg/6 h | Injection | - | Prevention and/or treatment of sepsis |
| (27) | In-vitro | B2, B9 | - | - | - | Stimulation of Mucosal-associated invariant cells |
| (28) | Review | B2 | - | - | - | Mucosal-associated invariant cells activation |
| (29) | Review | B3 | - | - | - | Boosts neutrophil counts |
| (30) | Review | B3 | - | - | - | Increases in neutrophil numbers |
| (31) | Review | B6 | - | - | - | Regulation of Immune Responses |
| (32) | Human study | B6 | 3, 15, 22.5, 33.75 μg /body weight/ day | - | 4 | Regulate IL-2 production and lymphocytes proliferation |
| (33) | Review | B6 | - | - | - | Thymic Epithelial Cells and T cells Differentiation |
| (34) | Animal study | B6 | 0.12 mg, and 120 mg/kg diets | Orally | 70 | Regulation of T- cells differentiation, IL-2 and IL-4 secretion |
| (35) | In-vitro and In Vivo | B9 | - | Orally | 90 | Regulation of T- cells |
| (36) | Review | B9 | - | - | - | Regulates gut immune system |
| (37) | Human study | B12 | 500 µg/day | Injection | 14 | Augmentation of CD8+ T cells and natural killer (NK) cell activity |
| (38) | Review | A, Bs, C, D, E | - | - | - | Support the protective activities of the immune cells |
| (39) | Virtual screening | B12 | - | - | - | Enhance the immune system |
| (40) | Review | C | - | - | - | Prevent and treat respiratory and systemic infections |
| (41) | Review | C | - | - | - | Shorten the duration of respiratory tract infections |
| (42) | Review | C | - | - | - | Enhance the immune system |
| (43) | Review | C | 50 mg/kg bw every 6 hours | Injection | 4 | Regulate inflammatory mediators |
| (44) | Review | D | - | - | - | Modulator of the immune system |
| (45) | Review | D | - | - | - | Modulation of innate and autoimmunity |
| (46) | Review | D | - | - | - | Enhance the immune system |
| (47) | Review | D | - | - | - | Barriers integrity, enhance chemo taxis, regulate inflammation |
| (48) | Review | D | - | - | - | Regulation of normal numbers of regulatory T-cells |
| (49) | Review | D | - | - | - | Modulate immune function and reduce the risk of infection |
| (50) | Review | D | - | - | - | Enhance the immune system |
| (51) | Review | E | - | - | - | Enhancing immunity in viral infections |
| (52) | Review | E | - | - | - | Stimulation of the immune system functions |
| (53) | Review | E | - | - | - | Enhance T- and B-cell-mediated functions |
| (54) | Review | E | - | - | - | Reduce the risk of infection and Modulate immune function |
| (55) | Human study | E | 400 mg | Orally | 30 | Enhances cell-mediated immunity |
| (56) | Review | E | - | - | - | Enhance the immune system |
| (57) | Review | E | - | - | - | Modulate immune function |
3. Results
Reviewed articles had been categorized to 5 main vitamin groups and 7 subgroups for vitamin B family groups according to their studied vitamins. The most studied vitamin group because of its enhancing activities on immune system, among whole of vitamins groups, had been identified as the group B vitamins (42.18%) and vitamin A, D, and E (26.66%, 22.22% and 13.33%) had been ranked in next steps, respectively. But, in individually comparison of vitamins, vitamin A had been identified as the vitamin with maximum number of studies (26.66%). At the current time and according to our searches and published data, there are only a few available published studies about association between vitamins and prevention/treatment of COVID-19 infection that among them more than half of studies (66.66%) had been focused on immune-modulatory effects of vitamin D on prevention and or treatment of COVID-19 infection. Vitamin C (50%), E (33.33%) and A (16.66%) had been ranked in next levels based on number of related articles, respectively. For each vitamin, reviewed references numbers, differential percentages of studies among whole of reviewed studies, differential percentages of studies about association of vitamins with COVID-19 infection and brief explanation of mechanisms of vitamins effects on immune system for every vitamin are also reported in Table 2, and detailed data for each vitamin are mentioned in next subtitles.
| Vitamins Names | Reviewed References Numbers | Percentage of Studies Among Whole of Reviewed Studies | Percentage Studies About Association with COVID-19 | Mechanisms of Vitamins Effects on Immune System |
|---|---|---|---|---|
| Vitamin A | (13-23, 51) | 26.66% (12/45) | 16.66% (1/6) | Enhance cytotoxicity; T-cell proliferation; stimulation of IL-2 secretion and etc. |
| Group B vitamins | (24-39) | 42.18% (19/45) | 0% (0/6) | As below |
| Vitamin B1 | (24, 25) | 4.44% (2/45) | 0% (0/6) | Antioxidative effect; regulation of NF-κB; suppresses oxidative stress and etc. |
| Vitamin B2 | (25-28) | 8.88% (4/45) | 0% (0/6) | Reduce cytokines; reduce nitric oxide; T cells activation and etc. |
| Vitamin B3 | (25, 29, 30) | 6.66% (3/45) | 0% (0/6) | Reduce inflammation; reduction of TGF-β gene expression; regulation of plasma lipoproteins and etc. |
| Vitamin B6 | (31-34) | 8.88% (4/45) | 0% (0/6) | Proliferation and differentiation of lymphocytes; IL-2 synthesis; IL-4 regulation and etc. |
| Vitamin B9 | (25, 35, 36) | 6.66% (3/45) | 0% (0/6) | NF-κB regulation; Treg cells differentiation; Treg cells maintenance and etc. |
| Vitamin B12 | (37-39) | 6.66% (3/45) | 0% (0/6) | Regulation of cellular immunity; CD8+ cells regulation; Enhancement of natural killer cell system and etc. |
| Vitamin C | (40-43, 56) | 11.11% (5/45) | 50% (3/6) | Antioxidant activity; enzyme cofactor; regulation of B cells and T cells genes and etc. |
| Vitamin D | (14, 44-51, 56) | 22.22% (10/45) | 66.66% (4/6) | Cathelicidin up-regulation; activation of T cells; stimulation of antigen-presenting cells especially dendritic cells and etc. |
| Vitamin E | (52-57) | 13.33% (6/45) | 33.33% (2/6) | Ant oxidative effects; activation of T cells; suppression of prostaglandin E2 synthesis and etc. |
3.1. Vitamin A and Its Role on Immune System Stimulation
Retinol, the fat-soluble vitamin, had been reported to have important effects on the immune system including: inherent immunity, humoral immunity and specific immunity mediated by cells, adding to efficient integrity of the mucosal epithelium, which had been reported closely related to vitamin A (Vit A)and its deficiency may result in severe disorders in eye, respiratory and gastrointestinal immune systems and increasing of their susceptibility to common pathogens (13).
All-trans-retinol and retinyl esters or β-carotene are different known form of Vit A driven from foods (14-16). Common metabolites of Vit A had been identified to have important effects on adaptive immune responses (14). Retinoic acid effects on immune system had been strongly reported as: enhance cytotoxicity (17), T-cell proliferation (18), stimulation of Il-2 secretion, signaling in T cells (18), T cells functions. Vit A deficiency in mice had been also reported to be associated with severe defects in TH-cell activity (19).
Vit A administration against measles in malnourished children in developing countries had been approved to result in considerable decrease in deaths consequent to viral infection (20, 21). Vit A administration in cortisone-treated animals had been also reported to abolish the immunosuppressant effects of cortisone (22). Enhancing effect of Vit A supplementation on antibody titers in humans had been reported (23). Vit A had been also reported to augment: function of leukocytes, reduce susceptibility to infective factors like carcinogenic factors, increasing integrity of mucosal membrane which can act as first line against pathogens penetration (13).
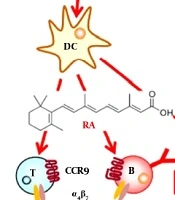
Retinoic acid, which is known as important metabolite of Vit A, is produced in gastrointestinal dendritic cells and has some unique roles including: overexpression of CC-chemokine receptor 9 (CCR9) and gut-homing receptors α4β7-integrin genes consequent to activation of lymphocytes (14), blockage of skin homing receptor’s up regulation and important intermediation in IgA-secreting B cell’s differentiation in gastrointestinal tract, especially in intestine. Retinoic acid had been also reported to have several roles on immune system including: potent stimulation activity on TH2-cells, considerable differentiation inhibition activity of TH17-cell’s, and development induction activity in TReg-cell (14). Figure 1 is obtained from Mora et al. (14) work in 2008 and shows effects of Vit A metabolites on gut mucosal immunity. Briefly, up-regulation of gut-homing receptors genes expressions and promote T-helper-2 (TH2)-cell differentiation had been identified as main mechanisms for effects of Vit A metabolites on gut mucosal immunity (14).
This figure is obtained from other study (14) and shows effects of vitamin A metabolites on gut mucosal immunity. A, In addition to upregulating the expression of gut-homing receptors, retinoic acid has also been reported to promote T-helper-2 (TH2)-cell differentiation. Moreover, retinoic acid blocks the differentiation of T helper 17 (TH17) cells and induces forkhead box protein 3 (FOXP3)+ regulatory T (TReg) cells in the presence of transforming growth factor-β (TGFβ) by reciprocally downregulating receptor-related orphan receptor-γt (RORγt) and inducing FOXP3 expression in T cells, respectively. Retinoic acid also enhances the TGFβ-driven induction of TReg cells and induces gut-homing receptor expression in both naturally occurring and induced TReg cells. TH17-cell differentiation requires TGFβ, interleukin-6 (IL-6), IL-23 and, in humans, IL-1β; B, B cells activated in non-mucosal lymphoid tissues, such as peripheral lymph nodes and spleen, mostly become IgG+ antibody-secreting cells (ASCs) and home to the bone marrow and sites of inflammation. By contrast, B cells activated in mucosal-associated lymphoid tissues (MALT) give rise to IgA+ ASCs. In MALT (including the gut-associated lymphoid tissue; GALT), TGFβ and CD40 ligand (CD40L) are essential for the generation of T-cell-dependent IgA responses, whereas BAFF (B-cell-activating factor) and APRIL (a proliferation-inducing ligand) are important for T-cell-independent IgA responses. APRIL is induced by Toll-like receptor (TLR) signals, commensal flora and thymic stromal lymphopoietin (TSLP). Inducible nitric oxide synthase (iNOS), which is also upregulated by TLR signals and commensal flora, produces nitric oxide (NO), allows proper TGFβ signalling and induces the production of APRIL and BAFF by dendritic cells. Thus, iNOS and NO are essential for both T-cell-dependent and -independent IgA responses. In the GALT, retinoic acid might contribute directly to the differentiation of T-cell-independent (and probably also T-cell-dependent) IgA+ ASCs. In addition, retinoic acid might contribute indirectly to T-cell-dependent and -independent IgA responses by inducing iNOS expression (14).
Considering the mentioned points Vit A can be considered as an important factor in enhancement of immune system and during the viral infections including COVID-19 it can improve the immunity state and act as potent preventive factor. Unfortunately, at the current time there is no study/report on effects of Vit A supplements in prevention and/or healing of COVID-19 infection. Animal and/or trial study on COVID-19 infected patients can be considered as novel topic for future studies and it is highly recommended to plain animal and/or in vivo studies on its effects on respiratory and/or mucous cell’s reconstruction.
3.2. Vitamin B Group and Their Role on Immune System Stimulation
3.2.1. Vitamin B1 (Thiamin)
It had been identified that tricarboxylic acid cycle is used by different kind of immune system cells including: Peyer’s patches dominant cells, which are naive B cells and don’t use from glycolysis pathways, and dominant immune cells in the intestinal lamina propria, which are IgA+ plasma cells and using glycolysis pathways. Vit B1 dependency is the main reason of this different metabolic property. Role of Vit B1 in tricarboxylic acid cycle as an essential and important cofactor is strongly approved (24). Disturbed/ insufficient performance of the tricarboxylic acid cycle consequent to Vit B1 deficiency had been reported to result in considerable decrease of the naive B cells number in peyer’s patches. In contrast by this decrease number of IgA+ plasma cells in the intestinal lamina propria remain normal. Effect of absorbed Vit B1 from diets on maintenance of naive B cells is required in order to efficient immune responses against antigens received from diets (24). Briefly, Vit B1 depletion decreases naive B cells without affecting IgA+ plasma cells via impairing of tricarboxylic acid cycle activity and finally impairs initiation of antigen-specific antibody responses (24). Antioxidative effect of Vit B1, suppression of oxidative stress and NF-κB regulation had been reported as important roles of Vit B1 on immune system (25). Considering the mentioned role of Vit B1, study on its effect in COVID-19 infected patients highly recommended for future works.
3.2.2. Vitamin B2 (Riboflavin)
There are little data about effects of Vit B2 on immune system and/or infections, but even giving to this subject, its role can be considered as an important factor in immune system stimulation. Intravenous administration of pure Vit B2 supplement against bacterial infection and induced shock subsequent to bacterial lipopolysaccharide had been studied in mice (26). It had been strongly approved that intravenous administration of Vit B2 can result in considerable decrease in pre-inflammatory cytokines, same as valuable reduce in nitric oxide, which has been induced by bacterial infection and their lipopolysaccharide (26). This subject highly approves that intravenous administration of pure Vit B2 can be recommended as potent choice in prevention and/or treatment of any kind of sepsis (26). Different immune function for Vit B2 (riboflavin) had been reported by Kjer-Nielsen et al. (27) and it had been suggested that molecules which are synthesized subsequent to metabolism of Vit Bs by bacteria may result in activation of a T cell called mucosa-associated invariant T (MAIT) cells, which are a unique class of immune cells (25, 27, 28). So, probable role of Vit B2 on immune system efficacy can be considered as a new topic especially in COVID-19 infected patients.
3.2.3. Vitamin B3 (Niacin)
It had been reported that an increase in the neutrophils number may be resulted via cellular pathways which have need to Vit B3 (29). Also it had been reported that Vit B3 administration in healthy peoples can result in reversible increase of neutrophil count (30). It is approved that after cessation of Vit B3 administration, neutrophil count become normal (30). Considerable increase in nicotinamide adenine dinucleotide’s (NAD+), CCAAT/ enhancer-binding protein-α (C/EBPα), C/EBPβ and the granulocyte colony-stimulating factor receptor can be also occurred after the oral administration of Vit B3, similar to cell’s treatment condition by granulocyte colony-stimulating factor (30). Reducing effects of inflammation status, inhibitory effects on NF-κB performance, reducing effects on expression of TGF-β gene and regulation of plasma lipoproteins had been identified for Vit B3adminstration (25). Although there are little data about role of Vit B3 on immune system’s stimulation and there is no report on its effect on COVID-19 infection, but it is highly suspected that this vitamin has augmentation role on immune system’s accuracy via mentioned effect. In vitro, in vivo and/or trial studies on Vit B3’s effect on immune system, especially in COVID-19 infected cells/patients can be considered as new topic for future studies.
3.2.4. Vitamin B6 (Pyridoxine)
Vit B6 had been reported as an essential factor for maintenance of lymphoid tissues and their immunological functions including: different kind of cell-mediated reactions and anti-bodies related reactions (31). Lymphoid tissue atrophy, consistent depression in primary antibodies response and secondary antibodies response, reduced lymphocyte number, decrease in delayed form of dermal hypersensitivity, reduced cytotoxicity, increase in survive of allograft, and etc. had been reported as common signs of immune system consequent to Vit B6 deficiency (31), and it had been reported that effects of Vit B6 deficiency on immune cells and their specific activities is more than other member of Vit B family and maybe it is occurred due to more sensitivity of immune cells to Vit B6 deficiency (31).
It had been also reported that Vit B6 depletion significantly result in reversible decrease in differential ant whole count of lymphocytes, interleukin 2 synthesis and response of lymphocytes in peripheral circulation to T cell and B cell’s mitogens (32). Also, effects of vitamin B6 on thymic epithelial cells, which are responsible for inducing T lymphocyte differentiation, and T lymphocyte differentiation had been studied and it had been reported that vitamin B6 deficiency can results in a reversible reduction of T lymphocyte numbers and defects of cellular immunocompetence (33). Results of a recent study highly approve that Vit B6 could benefit immunity of the organism and its deficiency influenced the immunity in organisms, it had been also reported that reduced value of growth rate, decreased amount of proliferation and differentiation of lymphocytes, reduced amount of interleukin 2 synthesis, elevated amount of interleukin 4 synthesis are detectable consequent to Vit B6 deficiency, but it has not any detectable effect on IFN-γ (34). To the best of authors knowledge and current time, there is no data/reported experiment on effect of Vit B6 in COVID-19 infected patients and study in this area is highly recommended for future studies.
3.2.5. Vitamin B9 (Folic Acid)
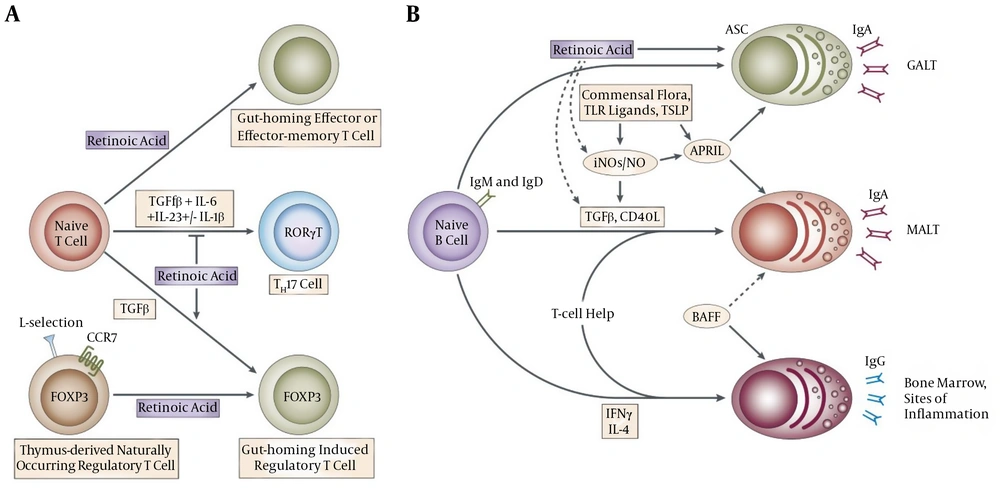
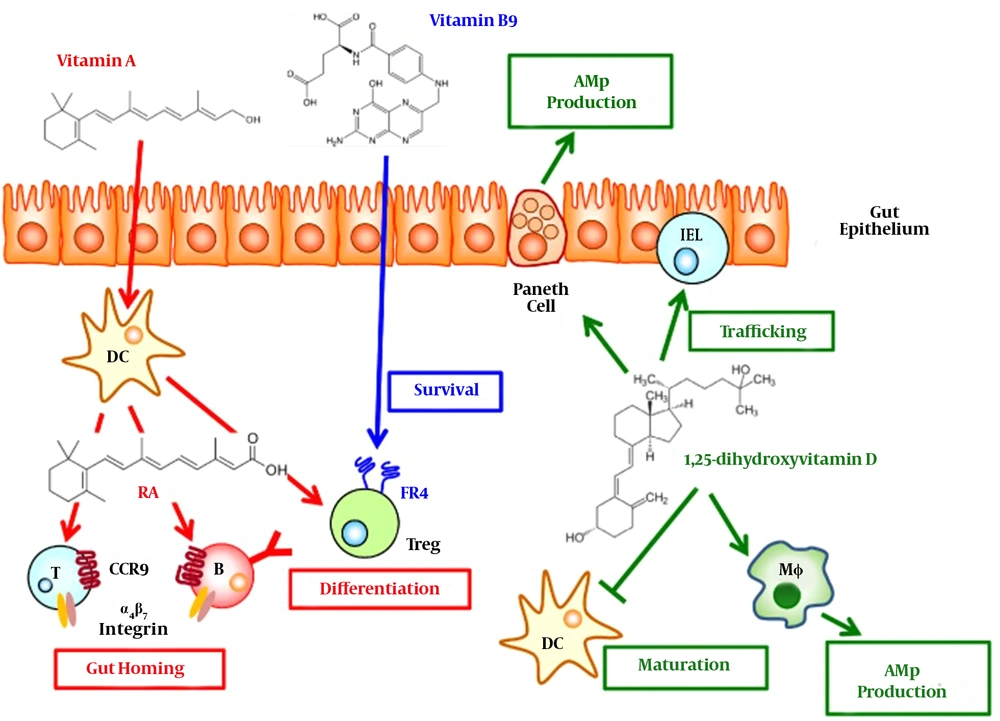
Regulation of immune responses and inhibition of homocystein induced NF-κB activation in cultured human monocytes had been reported as important roles of Vit B9 (25). Vit B9 had been identified as an important regulatory factor for survival of T (Treg) cells, which is responsible for expression of folate receptor 4 (special receptor of Vit B9). It had been also identified that in Vit B9 in vitro deficiency condition, Treg cells which are differentiated from naive T cells cannot be survive for long time; this impaired survival of Treg cells had been strongly reported to be highly related to reduced amounts of anti-apoptotic Bcl2 without any relation to interleukin 2. It had been also identified that decreased amount of Treg cells in gastrointestinal tract especially in small intestine, as a main location of Vit B9 absorption from diets, can be occurred consequent to Vit B9 in vivo deficiency (35). Figure 2 is obtained from other study (36) and shows vitamin‐mediated immune regulations in the gut. Unfortunately, although there is some study which highly recommends using Vit B9 during the COVID-19 infection but there is no available data/report on effect of Vit B9 on COVID-19 infected patients. So, study in this area is highly recommended.
This figure is obtained from other study (36) and shows vitamin‐mediated immune regulations in the gut. Vitamin A from diet is converted into retinoic acid (RA) by dendritic cells (DCs). B and T cells primed by DCs in the presence of RA express gut homing molecules (α4β7 integrin and CCR9). RA also promotes the differentiation of naïve T cells into regulatory T (Treg) cells. Upon the differentiation into the Treg cells, Treg cells start to express folate receptor 4 (FR4), a receptor for vitamin B9. The vitamin B9-FR4 axis is required for the survival of Treg cells. Vitamin D acts on Paneth cells on the epithelial layer and macrophages (MΦ) and aids production of antimicrobial peptides (AMPs). It also promotes the trafficking of intraepithelial lymphocyte (IEL) population and inhibits the maturation of DCs (36).
3.2.6. Vitamin B12 (Cobalamin)
Vit B12 had been reported to have an important role in cellular immunity, especially related to CD8+ cells and the natural killer cell system, which has effects on cytotoxic cells, it had been also identified that Vit B12 acts as an immunomodulator for cellular immunity (37). Support of body’s natural barriers including skin and mucosa, humoral immunity and production of anti-bodies and cellular immunity are three different levels which micronutrient (vitamins, trace elements) including Vit B12 helps immune system to work adequately, they are working altogether as a complex and their effects are synergy for other one (38). There is a little data about effects of Vit B12 on COVID-19 infection and it had been only reported that combination of ribavirin, telbivudine, Vit B12 and nicotinamide can be administrated for COVID-19 treatment, but unfortunately, mechanisms of their effects and other detailed data are not described clearly (39). In vitro, in vivo and/or trial studies on Vit B12’s effect on immune system, especially in COVID-19 infected cells/patients can be considered as new topic for future studies.
3.3. Vit C (Ascorbic Acid) and Its Role on Immune System Stimulation
Vit C had been introduced as an effective reducing agent which is known as antioxidant and/or enzyme cofactor, too. Effect of Vit C on lymphocytes is not completely clear, but it had been identified that Vit C regulates genes which are responsible for B cells and T cells generation and enhances their differentiation similar to their proliferation. Impaired immunity and higher susceptibility to infections had been also reported subsequent to Vit C deficiency and supplementation with vitamin C had been appeared to be useful in prevention and/or treatment of severe respiratory and systemic infections like COVID-19 infection (40). Adequate intakes of vitamin C had been strongly recommended to treatment of respiratory system infections including pneumonia, gastrointestinal tract’s disorders and infections including diarrhea and other infections like malaria via decreasing in common signs and decreasing of infection’s duration and finally decreasing of their incidences, especially in children in developing countries (41). Vit C had been also identified to improve strength of immune system via collagen synthesis enhancement, reducing the ROS and damage caused by it and also increases healing process, acting as antioxidant and increasing microbial killing through cytokines production (40, 42). It had been also strongly approved that intravenous administration of high-dose vitamin C can be useful in the treatment of sepsis and septic shock maybe due to its immunosuppressive effects. High-dose Vit C treatment acts as a pro-oxidant for immune cells, but as an antioxidant for lung epithelial cells, so Vit C treatment may protect innate immunity of alveolar epithelial type II through the inhibition of the lactate secretion, produced by the activated immune cells (43). Although there is a little data about recommendation of Vit C administration in COVID-19 infection, but there is no report/study focused on Vit C effects in COVID-19 infection, so very well focused studies in this area can be considered as hot topics for future studies.
3.4. Vit D and Its Role on Immune System Stimulation
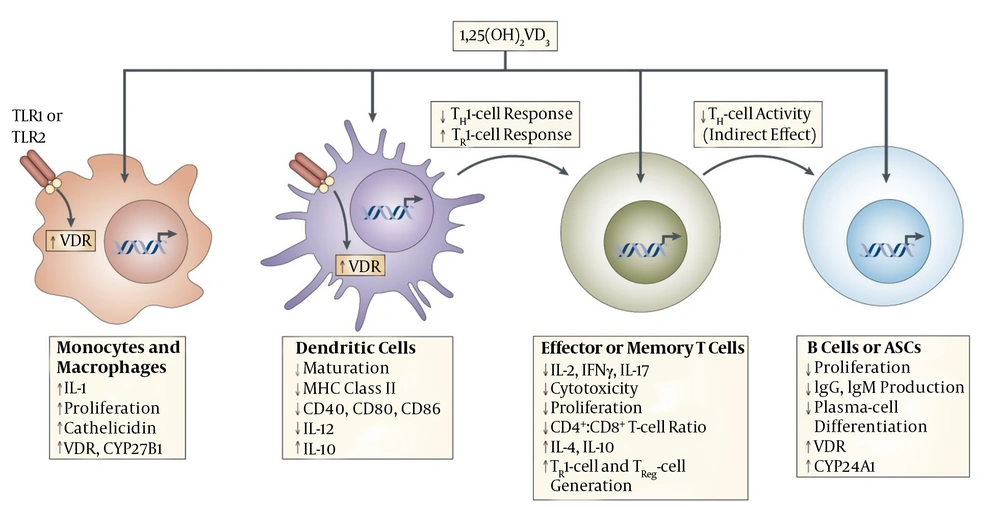
The 1,25-dihydroxy Vit D3 (1,25(OH)2D3) had been identified as the active form of Vit D, adding to its roles in regulation of calcium and phosphorus metabolisms and bone-formation, it had been identified as an immunomodulator influencing different immune cells. It had been also identified that adding to Vit D3 role in modulation of immune system via affecting the monocytes, macrophages, dendritic cells, as well as T-cell and B-cell, it had important effects on innate and adaptive immune responses, too (44). It is identified that cathelicidin, an anti-microbial peptides which enhances clearance of bacteria in immune cells adding to other sites, is highly up-regulated by hormonal form of Vit D. Figure 3 had been obtained from other study (14) and shows the detailed mechanisms of vitamin D immunomodulation (14). The Vit D modulation effects on the adaptive immune system had been identified to occur via its direct enhancing effects on; activation of T cells, stimulation of antigen-presenting cells especially dendritic cells (45).
Crohn’s disease, eczema, psoriasis, juvenile diabetes mellitus, asthma, multiple sclerosis, and rheumatoid arthritis, which are some important autoimmune diseases, had been identified to be closely associated with Vit D and its deficiency, also adding to its benefaction in treatment of tuberculosis, common infections of chest, urinary tract, eye and wounds, it had been strongly approved in treatment of influenza (46, 47), and it had been also identified to be highly supportive in treatment of different infections including: viral, bacterial and fungal infections (47). Also, gastrointestinal tract autoimmunity disorders consequent to valuable increase in IL-17 and IFN-γ secreting T-cells numbers and a concomitant reduction in regulatory T-cells had been highly approved to occur due to absence of Vit D and its receptors (48).
Risk of lungs infections including pneumonia can be reduced after administration of Vit D, and it may occur through several mechanisms including: inducing cathelicidins and defensins, which may result in reduced amount of: replication rates of viruses and concentration of inflammatory cytokines, in contrast by considerable increased values of anti-inflammatory cytokines concentrations (49). In a recent published study, in order to reducing the risk of infection, it had been strongly recommended that peoples who are at the risk of influenza and/or COVID-19 infection must receive specified amount of Vit D and continue to its reception according to the defined protocol for prevention, and higher dosages of Vit D3 might be useful in order to treatment of COVID-19 infected patients (49). In a recent published study, association between mortality rate of COVID-19 infection and geographical latitude had been studied and possible relationship between Vit D deficiency and severity of COVID-19 infection had been strongly approved. This relationship had been reported more stronger when comparison between Scandinavian countries (with considerable decreased rates of Vit D deficiency and low rate of COVID-19 mortality rate in their countries) and nations which are located in low latitudes (with considerable values of Vit D deficiency and high rate of COVID-19 mortality rate such as Indonesia and the Philippines) had been done and its detailed data had been reported (50). In a recent published article effect of Vit D and A on probable prevention of COVID-19 infection had been reviewed by authors (51). Although there are some data about association between COVID-19 infection and Vit D, but there is no available study which can approve that how much this association is considerable, so clinical trials and in vivo and/or in vitro studies in this area are highly recommended.
This picture is obtained from other study (14) and shows the mechanisms of vitamin D immunomodulation. Systemic or locally produced 1,25(OH)2VD3 exerts its effects on several immune-cell types, including macrophages, dendritic cells (DCs), T and B cells. Macrophages and DCs constitutively express vitamin D receptor (VDR), whereas VDR expression in T cells is only upregulated following activation. In macrophages and monocytes, 1,25(OH)2VD3 positively influences its own effects by increasing the expression of VDR and the cytochrome P450 protein CYP27B1. Certain Toll-like-receptor (TLR)-mediated signals can also increase the expression of VDR. 1,25(OH)2VD3 also induces monocyte proliferation and the expression of interleukin-1 (IL-1) and cathelicidin (an antimicrobial peptide) by macrophages, thereby contributing to innate immune responses to some bacteria. 1,25(OH)2VD3 decreases DC maturation, inhibiting upregulation of the expression of MHC class II, CD40, CD80 and CD86. In addition, it decreases IL-12 production by DCs while inducing the production of IL-10. In T cells, 1,25(OH)2VD3 decreases the production of IL-2, IL-17 and interferon-γ (IFNγ) and attenuates the cytotoxic activity and proliferation of CD4+ and CD8+ T cells. 1,25(OH)2VD3 might also promote the development of forkhead box protein 3 (FOXP3)+ regulatory T (TReg) cells and IL-10-producing T regulatory type 1 (TR1) cells. Finally, 1,25(OH)2VD3 blocks B-cell proliferation, plasma-cell differentiation and immunoglobulin production. ASCs, antibody-secreting cells (14).
3.6. Vit E and Its Role on Immune System Stimulation
Protection of cell membrane against oxidative damages is one of most important functions of Vit E in the body (52), and because of the fact that immune system cells are especially in the high risk of oxidative damages, Vit E concentration in the membranes of immune system cells is high. Totally, Vit E is identified as an important essential factor for normal function of the immune system, and its deficiency may result in; diminish the immune system ability against important infective microorganisms, production of delayed-type hypersensitivity (DTH) reaction, or to mount an antibody response to antigen (52).
Interference in immature T cells differentiations in thymus had been reported as an important function of Vit E, and its deficiency may result in early reduce of cellular immunity simultaneous by aging consequent to considerable decreased value of differentiation in immature T cells (53). Vit E had been reported to modulate immune system by different mechanisms including: its stimulation effects on activation of T cells and its suppressor effects on prostaglandin E2 synthesis. It had been also identified that prostaglandin E2 is an important T cell-suppressing lipid mediator which become increased consequent to aging process (54). Short-term Vit E supplementation had been also approved to improve the immune system functions in elder hood via its considerable effects on different lipid-peroxidation products such as prostaglandin E2 (55). Vit E deficiency had been rarely reported in humankind and enhancement of T cell’s performances consequent to increased amount of Vit E intake, even more than its recommended intake amounts, especially in elder hood in both of elder humans and other mammals (54). Elevated values of resistance of respiratory system against common pathogens in old humans and old mice had been also reported to be highly associated to Vit E supplementation in elder hood (54). In a recent review study, administration of Vit E, D, and C had been also recommended in prevention of COVID-19 infection according to the result of animal studies (56). Unfortunately, although it had been recommended that administration of some nutrient supplementations such as vitamin C, vitamin E, selenium, and zinc may have beneficial effects on patients with COVID-19 (57), but to the best of authors knowledge there is no available data about effect of Vit E administration on COVID-19 infection and study in this area is highly recommended for future study.
4. Conclusions
Briefly, current study as the first study in this way, reviews the roles of vitamins including: whole of the water soluble and fat soluble vitamins, on immune system and prevention and/or treatment of COVID-19 infection based on available published data. This study shows that among whole of vitamins, roles of Vit A, C and D are more defined and maybe more effective on immune system and most of current studies and available preventive and/or treatment protocols for COVID-19 are highly concentrated on these vitamins. In vitro, in vivo, clinical trials and any kind of studies/reviews about role of vitamins on prevention and/or treatment of COVID-19 infection can be strongly considered as novel topics for future studies. At the current time and regarding to available data, result of current review emphasizes on the importance of vitamins in prevention of viral infections including COVID-19 and based on current review’s results, sufficient vitamin intake can be recommended in order to prevention from viral infections including COVID-19 infection.