1. Background
Although medications have several benefits, can cause significant harm to a potentially high number of people, and adverse drug reactions (ADRs) play an important role in causing such harm. ADRs are the leading cause of mortality and morbidity (1). World Health Organization (WHO) defined the ADR as a noxious and unintended response to a drug at normal doses (2), which is responsible for an extended length of hospital stay and augmented economic burden (1). In reaction to the thalidomide tragedy of the 1960s, when nearly 10,000 babies were born with deformities due to the adverse effects of thalidomide, the WHO Program for International Drug Monitoring came into effect in 1968 (3).
Globally, adverse reactions to medications are the fourth to the sixth leading cause of death. ADRs have become a major global public health concern that needs to be addressed at all levels of the health care system. In January 2000, the Institute of Medicine reported that medication-related problems annually cause nearly 44,000 - 98,000 deaths. In which an estimated 7,000 deaths are attributed to ADRs. Throughout the United Kingdom, about 6.5% of all hospital admissions and 0.15% of all deaths are due to ADRs (4). A study conducted in the UK found that 15.8% of the hospitalized patients developed ADRs (5). In the United States, each year, more than 100,000 people die due to significant adverse drug reactions (1). Another US research reported that 6.3% of hospitalized patients were admitted as a direct result of an ADR. With enhanced prescribing, management, monitoring, and enforcement, more than half of the ADRs can be prevented. For example, in Australia, the medication error is a major contributor to medical errors (26% of the 27,000 medication-related incidents) (6). The intensive monitoring study of ADRs conducted in Jeddah (Saudi Arabia) also reported a substantially high prevalence of ADRs, mainly due to medication errors (7).
In most of the cases, ADRs are extremely harmful and may be fatal. Avoiding ADR requires a thorough knowledge about how they can be monitored, controlled, and reported. An ADR monitoring and reporting program provides information on the quality and safety of pharmaceutical products, helps in implementing risk reduction strategies, avoids expected adverse effects, and help track the occurrence of ADRs. Besides, such programs are useful for training health staff, patients, pharmacists, and nurses on adverse effects of drugs and enhancing awareness about ADRs (8). Collecting information about the possible hazards of drugs is vital to address the drug-induced disease concerns. A failure to maintain continuous vigilance of medicines in patients may lead to serious and sometimes life-threatening effects (6). There should be a tendency not only to observe but also to track unnecessary and unforeseen medical incidents in all places where medicines are used. The adverse drug reactions or adverse effects may occur at any dosage and by overdose or misuse or abuse of medicine (9). The monitoring process should include all aspects of the healthcare system, such as public and private hospitals, general practitioners, nurses, retail pharmacies, and pharmacists. Wherever medicinal products are used, they should be ready to observe and report unwanted adverse events (10).
It is a long time that countries such as the USA and the UK apply “pharmacovigilance”, but couldn’t significantly decrease the incidence of ADRs yet. Whereas, in Pakistan, pharmacovigilance (PV) is in its initial phase. In 2012, more than 100 patients were killed by a counterfeit antihypertensive medication at the Punjab Cardiology Institute (PIC) hospital in Lahore, Pakistan (11). Shortly after the PIC scam, the Government of the Punjab Province (Pakistan) opted for several short-term and long-term strategies and finally decided to establish the WHO-recommended Pharmacovigilance Centre in Pakistan (9). The pharmacovigilance system in Pakistan is still in the development stage, due to lack of knowledge, indifference, or lack of preparation because very few research on the ADR system is performed in the past (12). There is no central database for ADRs in Pakistan. Also, no ADR is registered to the WHO database in prior years. Public health programs do not perform ADR tests (13).
Successful prevention of ADRs requires increasing the awareness of all health care providers as well as society. This study acknowledges the necessity and importance of ADR reporting and monitoring and hopefully will highlight the steps that must be taken to incorporate the concept of pharmacovigilance.
2. Objectives
The prime purpose of the current study was to assess the knowledge, attitude, practices, and perceptions of the health care professionals (pharmacists, physicians, and nurses working in the hospitals of Lahore) concerning the ADR monitoring and reporting in Lahore, Pakistan.
3. Methods
3.1. Study Design, Settings, and Subjects
This is a prospective, cross-sectional analysis that was conducted in one of Pakistan’s largest cities; Lahore. The ethical approval of the study was obtained from the Humans Ethics Committee, University of the Punjab, Lahore, Pakistan (HEC/PUCP/1942A). The study commenced from October 2018 to December 2018. The sample size was calculated based on the total number of tertiary care hospitals in Lahore city. Using the convenience sampling method, 150 healthcare professionals (HCPs) were randomly selected among fifty physicians, fifty pharmacists, and fifty nurses with an acceptance rate of 78%, 80%, and 92%, respectively. Each specialist was asked to fill out a manually administered pre-validated questionnaire. The participants were working in different hospitals (public and private). Thirty hospitals were covered which represented 52% of the total hospitals in Lahore.
3.2. Questionnaire
The face and content validity of the questionnaire was evaluated by three experts in the field of pharmacy from the University of Balochistan, Quetta, Pakistan, and the University of the Punjab, Lahore, Pakistan. A pilot study was conducted on 30 participants, and the questionnaire was adjusted accordingly for the items. The final form contained six questions on demographics, six on pharmacovigilance, 13 on knowledge, nine on attitude, 11 on encouraging and discouraging factors, 10 on practices, and six on perceptions. These questions covered the main areas of interest. Reliability was determined by Cronbach alpha for 55 items of the questionnaire, which yielded a value of 0.76.
3.3. Statistical Analysis
Data were analyzed using SPSS version 20. Descriptive statistics were used to obtain the frequencies and percentages. To check the association between having the concept of pharmacovigilance among different professions, the chi-square test was used.
4. Results
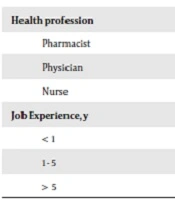
A total of 150 pharmacists, physicians, and nurses were approached, out of whom 40, 39, and 46 accepted to participate, respectively. 125 (65%) of them were female. In terms of working experience, 25.6% had more than 5 years of experience, while 20% had less than one year of experience, and the remaining had 1 - 5 years of experience (Table 1).
| Values | |
|---|---|
| Gender | |
| Male | 43 (34.4) |
| Female | 82 (65.6) |
| Health profession | |
| Pharmacist | 40 (32.0) |
| Physician | 39 (31.2) |
| Nurse | 46 (36.8) |
| Job Experience, y | |
| < 1 | 25 (20.0) |
| 1 - 5 | 68 (54.4) |
| > 5 | 32 (25.6) |
aValues are expressed as No. (%).
The questionnaire contained some open-ended questions where pharmacists, nurses, and physicians were asked to define different terms for them. When asked for the correct definition of “pharmacovigilance”, 95, 17.3, and 58.9% personnel respectively defined the term correctly. Similarly, when asked about the definition of “ADRs”, 70, 10, and 30.5% knew the correct definition, respectively. Only 18 (out of 150) practitioners had information about the Vigibase. 70% pharmacists, 32.43% physicians, and 4.34% of nurses were aware of the available pharmacovigilance program at the provincial level. When inquired about the body responsible for monitoring ADRs in Pakistan, 65% pharmacists, 84.61% physicians, and 95.65% of nurses gave correct answers. The Pearson chi-square showed a significant difference between healthcare professionals about the concept of pharmacovigilance (P < 0.01), Table 2.
| Health Professionals | ||||
|---|---|---|---|---|
| Pharmacist | Physician | Nurse | P Value | |
| Concept of pharmacovigilance | ||||
| Yes | 40 (100) | 23 (58.97) | 11 (23.9) | < 0.001 |
| The correct definition of Pharmacovigilance | ||||
| Know correct | 38 (95) | 23 (58.97) | 8 (17.39) | < 0.001 |
| Pharmacovigilance Center in Pakistan | ||||
| Yes | 26 (65) | 13 (33.33) | 4 (8.69) | < 0.001 |
| Definition of ADR | ||||
| Yes | 28 (70) | 11 (28.20) | 5 (10.86) | < 0.001 |
| Knowledge of ADR Reporting Organization in Pakistan | ||||
| Agree | 30 (75) | 14 (35.8) | 21 (45.65) | < 0.001 |
| Total | 40 | 39 | 46 | < 0.001 |
aValues are expressed as No. (%).
Our survey on the attitude and practices of health personnel towards ADR monitoring and reporting revealed that 87% of pharmacists, 82.5% of physicians, and 82.6% of nurses had a history of ADR identification in patients. Out of which, 52, 41, and 19% of pharmacists, physicians, and nurses have a history of reporting ADR, respectively. Among all health professionals, 75% of pharmacists, 48% of physicians, and 41% of nurses knew the period within which serious ADRs experienced by a patient should be reported. When asked about the preferred mode for reporting ADRs, 57% of pharmacists, 56% of physicians, and 50% of nurses mentioned to “phone call to the drug company” as the best medium to report ADR. The least preferred method was verbal information to the drug company routinely.
Factors that could prevent health professionals from recording ADRs were also assessed. Three main reasons for stopping health providers from recording ADRs were “not having enough information about the patient” (57% pharmacists, 79% physicians, 78% nurses). Most physicians (69%) mentioned to “fear of legal liability”, while 60% of nurses and 27% of pharmacists (27%) mentioned this concern. The significant agreement of all three professionals was on “Unawareness about the existence of a national ADRs reporting system (78.4% of all respondents) (Table 3).
| Statements | Health Professionals | |||
|---|---|---|---|---|
| Pharmacist | Physicians | Nurses | P Value | |
| The reaction is unusual | ||||
| Agree | 38 (95) | 34 (87.17) | 26 (56.5) | 0.000 |
| The reaction is to a new product | ||||
| Agree | 36 (90) | 31 (79.4) | 46 (100) | 0.002 |
| Reaction not reported before for a particular drug | ||||
| Agree | 38 (95) | 37 (97.8) | 41 (89.1) | 0.145 |
| The reaction is well recognized for a particular drug | ||||
| Agree | 27 (95) | 32 (82.05) | 34 (73.9) | 0.162 |
| The reaction is of a serious nature | ||||
| Agree | 38 (95) | 39 (100) | 43 (93.4) | 0.050 |
| Level of clinical knowledge is not enough to decide whether or not an ADR has occurred | ||||
| Agree | 31 (77.5) | 24 (61.5) | 41 (89.1) | 0.020 |
| Uncertain association between the drug and the adverse reaction | ||||
| Agree | 27 (67.5) | 31 (79.4) | 39 (84.7) | 0.117 |
| No enough information available from the patient | ||||
| Agree | 23 (57.5) | 31 (79.4) | 36 (78.2) | 0.094 |
| Fear of legal liability | ||||
| Agree | 11 (27.5) | 27 (69.2) | 28 (60.8) | 0.000 |
| Unaware of the need to report an ADRs | ||||
| Agree | 26 (65) | 36 (92.3) | 39 (84.7) | 0.009 |
aValues are expressed as No. (%).
5. Discussion
The main purpose of this study was to assess the awareness, attitudes, and practices of pharmacists, physicians, and nurses employed in different hospitals (both public and private) of Lahore, Pakistan, concerning the ADR monitoring and reporting. Several studies are conducted in different countries on the ADR, but the current study is the first of its kind in Pakistan.
The current study showed that the majority of pharmacists have adequate knowledge about the principle of pharmacovigilance and ADRs, while other health personnel did not have such a level of knowledge (70: 10: 30 pharmacists: physicians: nurses). When it came to know about the authority responsible for monitoring ADRs and where they should be reported, pharmacists did not have sufficient information. When the participants were asked if they had any information about the pharmacovigilance program in their province, not all had a fair amount of ideas. Out of all three professional categories, only 4.3% of the nurses had familiarity with ADR reporting. A similar study on physicians, pharmacists, and nurses conducted in a teaching hospital in India concluded that 62.4% of health staff responded correctly to the pharmacovigilance concept. 75.2% of health staff were aware of the presence of India’s National Pharmacovigilance System (14). Another study conducted in India reported that 28.57% of health professionals, including 51.47% of medical and 8.64% of nursing professionals, were aware that the Central Drugs Standard Control Organization (CDSCO) is the regulatory agency responsible for controlling ADRs in India. Similarly, when evaluating healthcare professionals' awareness of pharmacovigilance, a median of 70.14% of medical and 69.13% of nursing professionals responded correctly to the pharmacovigilance concept (15).
The current study revealed a huge gap between the number of ADRs experienced by patients, and those identified and reported (87% of pharmacists, 82.5% of physicians, and 82.6% of nurses had a history of identifying an ADR, out of which 52, 41, and 19% of pharmacists, physicians, and nurses have a history of reporting an ADR, respectively. In line with the results of the current study, when our participants were asked about the factors that discouraged them from reporting ADRs, most of them mentioned to “fear of legal liabilities”, “lack of patient information”, and “unawareness” as the key factors.
A survey carried out in the United Arab Emirates revealed weak ADR reporting practices by respondents; only 19 and 12.1% of doctors and hospital pharmacists revealed ADRs, respectively (16). Another study concluded that, to the statement “had you ever reported an ADR to a PV center”, nearly three-quarters of the participants declared that they never reported any ADR to a PV center and 40.8% ascribed it to “non-availability of ADR forms at their sites” (17). The current study demonstrated the least number of ADR monitoring and reporting by health professionals. A possible justification behind such practices may be the lack of training of health professionals at the early stages of work. Another conceivable reason can be hospital policies that are centered on outcomes of the treatment, instead of unfavorable events that occur during the treatment.
In this research, factors that influenced and motivated health professionals to report ADRs were assessed. According to the findings, most of the professionals preferred to report serious reactions (but this was inconsistent with their actual actions), even most preferred to report rare reactions and reactions that had not been reported before. The discrepancy lies in the reporting of well recognized ADRs of a particular drug, where 67% of pharmacists, 82% of physicians, and 74% of nurses were encouraged to report such ADRs, while the rest did not consider it as an important factor. The present study identified the facilitators which motivate health care professionals to report ADRs. Most practitioners were inclined to report both common (well reported) and uncommon ADRs, contrary to a study conducted in Saudi Arabia, which reported reluctance by the health professionals to report already documented and well known adverse reactions as the most important obstacle to report ADRs (18). A research conducted in Germany found that serious unknown adverse drug reactions (81.1%), proven drug reactions (72.9%), and severe identified drug reactions (65.2%) were the most likely ADRs for documentation (19).
Based on the results, many healthcare professionals working in different hospitals in Lahore were aware of what adverse drug reactions, but never actually reported them. 59.2% of all practitioners responded that monitoring and reporting ADRs were not performed in their respective hospitals. The research also emphasized that there is a strong association between pharmacovigilance training and ADR reporting by health professionals and showed that the importance of tracking and recording adverse effects could be enhanced by the academic intervention (14). We propose that hospital managers, pharmaceutical companies, and drug regulatory authorities play a major role in training doctors to track and report ADRs. It is therefore suggested to include pharmacovigilance in the undergraduate curriculum of healthcare professionals and to establish a network of doctors for ADR reporting, easy access to ADR reporting forms, and promotion of patient self-reporting. Furthermore, a specific mandate imposed by the Ministry of Health (MOH), which includes ADR reporting as an official professional requirement for pharmacists, physicians, nurses, and other health professionals, may be useful in this respect (15).
5.1. Strengths and Limitations
The current study had limitations. First, the study was conducted in a few hospitals in Lahore City, hence, the results may not be generalized to the whole country. Second, the sample size was not quite large. Since pharmacists are key healthcare professionals in monitoring and reporting ADRs. A limited number of pharmacists were employed in hospitals, which made it impossible to include a large number of participants since the current study aimed to include an equal number of physicians, pharmacists, and nurses. Third, no formal sampling frame was available to choose the study participants, therefore, the convenience sampling method was adopted, which may not be the exact representation of the study population. However, this study is the first to report the knowledge, attitude, practices, and perceptions of health professionals about ADR monitoring and reporting and has yielded valuable information about the ADR and PV in Lahore, Pakistan.
5.2. Conclusions
The current study demonstrated poor results of the knowledge, attitude and practices (KAP) among health professionals working in the hospitals of Lahore, Pakistan related to ADR monitoring and reporting. Since most health professionals were motivated to report identified ADRs, it is the responsibility of the governing authorities to provide them with a suitably efficient platform to practice proper ADR reporting and monitoring. Educational campaigns and training, financial incentives, and the simplification of the reporting process can change the attitudes and practices of health professionals. Besides, making ADR reporting mandatory will raise awareness among health care professionals about the value of PV in Pakistan. With clear guidelines, targets in all healthcare settings will be aimed to transform the definition of healthcare positively to view ADR reporting as a widely agreed everyday activity.