1. Background
Multiple sclerosis (MS) is commonly regarded as an idiopathic, autoimmune inflammatory disease that destroys the central nervous system. MS has three characteristics: demyelination, inflammation, and gliosis (1, 2). Despite many advances in the field of etiology of MS, the exact cause of this disease is still unknown. However, epidemiologic and genetic studies have proposed a role for infectious pathogens in MS, from which only Epstein-Barr virus (EBV) is considered as a risk factor (3). Toxoplasma gondii is a common obligatory intracellular parasite that can cause a lifelong chronic infectious disease in the host (4). Also, some parasitic infections play several significant roles in the formation and worsening of autoimmune diseases. For example, the traces of toxoplasmosis have been noticed in many autoimmune diseases, such as autoimmune thyroid disease, systemic sclerosis, rheumatoid arthritis, and discoid lupus erythematosus (5-7). It seems that parasite and host antigens’ high resemblance can cause autoimmune disorders by a parasitic infection (7). To date, the exact effect of toxoplasmosis on the autoimmune disease is still doubtful. Studying this disease with the mentioned parasite is necessary due to the increasing occurrence of MS, the widespread and high prevalence of toxoplasmosis (8), and the complications arising from brain cysts caused by toxoplasmosis and inflammatory responses by the macrophage system.
2. Objectives
3. Methods
3.1. Study Population
One hundred and thirty patients were followed-up in the Department of neurology at Shiraz University of Medical Sciences, who were definitively diagnosed with MS between 2016 - 2018 in terms of the 2005 McDonald criteria (11). None of them showed any evidence of immunodeficiency or other immunologic abnormalities. The serum samples obtained from MS patients (n = 130) were collected along with a group of age and gender-matched controls (n = 130) with the similar socioeconomic status to the patient group in order to evaluate the prevalence of T. gondii IgG antibodies (ATXAb) in terms of the consensus definition of control groups in cerebrospinal fluid biomarker studies in MS from 2013 (12). The exclusion criteria were the patients who had a history of neurological illness, Alzheimer disease, Parkinson disease, schizophrenia, depression, migraine, epilepsy, brain surgery, earlier encephalitis/meningitis, head injury, substance abuse, clinical evidence of immunodeficiency, or other immunologic abnormalities.
3.2. Serological Assay
The blood serum of the individuals in the two groups was separated from their whole blood samples and stored at -20°C until serologic examination. The serum was diluted and added to ELISA kit vials (PishtazTeb, Iran) for the detection of ATXAb. The technique was performed based on the manufacturer’s instructions. The plentitude of toxoplasmosis was analyzed in the two groups via this technique and titration measurement of the immunoglobulin G antibody. The status of toxoplasmosis was determined according to the formula on the kit. The immune status ratio (ISR) value of each specimen was calculated by dividing the sample absorbance by the calibrator value based on the manufacturer’s guide. In this regard, the positive and negative results of each specimen were determined by dividing the optical density of each sample via the calibrator value based on the manufacturer’s guide. The results higher than 1.1 were interpreted as positive, less than 0.9 as negative, and between 0.9 and 1.1 as equivocal.
3.3. Statistical Analysis
The descriptive statistics were used to characterize the sample according to age, sex, seroprevalence, and average IgG antibody titer. For statistical analysis, SPSS 20 software was used (SPSS, inc., Chicago, USA). Moreover, the independent t-test was used to compare the age means for two groups. The rates of ATXAb positivity and gender of the MS patients and the control group subjects were compared by the chi-square test. The intensity of the IgG antibody in two groups of control and MS patients was calculated by an independent t-test. In addition, the normality of data was tested by the Kolmogorov-Smirnov test. Effect size (Cohen’s d) was calculated for pairwise comparison. Cohen offered the following guidelines to interpret the magnitude of the standardized mean difference SMD in the social sciences: small, SMD = 0.2; medium, SMD = 0.5; and large, SMD = 0.8 (13). A statistical significance level of α < 0.05 was considered.
4. Results
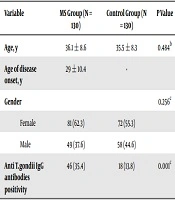
Out of 130 MS patients, 70 had relapsing-remitting MS, 30 primary progressive MS, 20 secondary progressive MS, and 10 subjects had progressive-relapsing MS. The mean age at the onset of the MS was 29 ± 10.4 years old (min = 17 and max = 50 years old). The average age of the MS patients and control subjects was 36.15 ± 8.66 and 5.50 ± 8.39 years old, respectively, which was not significantly different based on the t-test (P = 0.48). There were no statistically significant differences among the MS patients and ordinary individuals in terms of gender (P = 0.256). Table 1 shows the distribution of ATXAb in the two groups.
A significant proportion of the MS group received some forms of immunosuppressive and immunomodulatory therapies. We evaluated the impact of such chemotherapies on the frequency of ATXAb. The MS patients consisted of three following categories in terms of chemotherapy: untreated patients (n = 15), first-line disease-modifying drugs (n = 85), and patients who had received escalation therapy (n = 30). Accordingly, the ATXAb seropositivity for each group was 33.3%, 34.1%, and 36.6 %, respectively. Moreover, the difference in seropositivity rates among these three groups was not statistically significant (P > 0.05). Of 130 (35.4%) cases with MS and of 130 (13.8%) ordinary controls, 46 and 18 subjects were ATXAb positive, respectively. There was a statistically significant difference in terms of the rates of positivity between the MS patient group and the control group (P = 0.001) (Table 1). Table 2 shows the mean ATXAb concentrations among controls and MS patients. The mean ± standard deviation ATXAb levels in controls and MS patients were found to be 61 ± 34 and 114 ± 47 IU/mL, respectively, and statistically significant differences between the levels of two groups were founded (P = 0.001). Based on the effect size result (Cohen’s d was 1.2), the SMD was large.
5. Discussion
The results of the current study show that the prevalence of toxoplasmosis is significantly greater in MS patients than in the control group. Toxoplasmosis can be considered as a risk factor for MS patients. Previous studies investigated the relationship between MS and toxoplasmosis. In one of these studies, Pestechian et al. (14) analyzed the prevalence rates of parasitic infections, including toxoplasma, in MS patients. They showed that 18 out of 50 MS patients and 24 out of 50 healthy individuals were serologically positive as Toxoplasma. Unlike our results, they found no significant difference between the two groups that might be due to their sampling. They included family members of MS patients as the control group, so the likelihood of parasitic infection in the control group was almost similar to the MS group (14). Our findings were consistent with the findings of Oruc et al. (10), who found a direct relationship between the seropositivity of toxoplasmosis and MS. However, in contrast to our work, their sample size was very small, so we decided to use a larger sample size. Several studies demonstrated a relationship between toxoplasmosis and other autoimmune and neurodegenerative disorders such as lupus, Hashimoto’s thyroiditis, and Parkinson’s disease (PD) (5, 15-17). The present study analyzed the prevalence of T. gondii IgG antibodies, showing that ATXAb was significantly more in MS patients than the control group. Bach et al. (18) cited two main reasons for the stimulation of autoimmune diseases by parasitic infections. Accordingly, one mechanism is based on the molecular similarity between the antigens of these diseases and the antigens of the target organ (18). For example, a reciprocal reaction has been reported between antibodies that acts against toxoplasma and antigens of the malignant tissues of the cervix (19). Additionally, reactions between antibodies that act against Toxoplasma and thyroid organ have been observed in pregnant women. Also, based on the second mechanism, the activation of toll-like receptors of the infected person with toxoplasmosis can cause the increased production of autoimmune antibodies, which finally cause autoimmune diseases such as MS (18).
The results of the present study showed that female MS patients significantly outnumbered men. These results were in line with previous studies (20, 21). Lee et al. (22) reported that the prevalence of MS is higher among Canadian and Asian women living in Canada. However, in some studies, the rate of disability as a result of MS is the same among men and women (23).
The current study faced some limitations, such as small sample size and the need to evaluate the effect of chemotherapies in MS patients on the frequency of ATXAb with a larger population.
It is suggested that the pathomechanisms be clarified in the future. Also, further studies are necessary to be conducted in a larger population size to find the relationship between autoimmune diseases such as MS and toxoplasmosis. In conclusion, we demonstrated a higher seropositivity of toxoplasmosis in multiple sclerosis patients than the controls. This might provide insight into new mechanisms underlying the pathogenesis of MS. It could also improve the development of the potential immune-modulating therapies for MS as well as other autoimmune diseases.