Dear Editor,
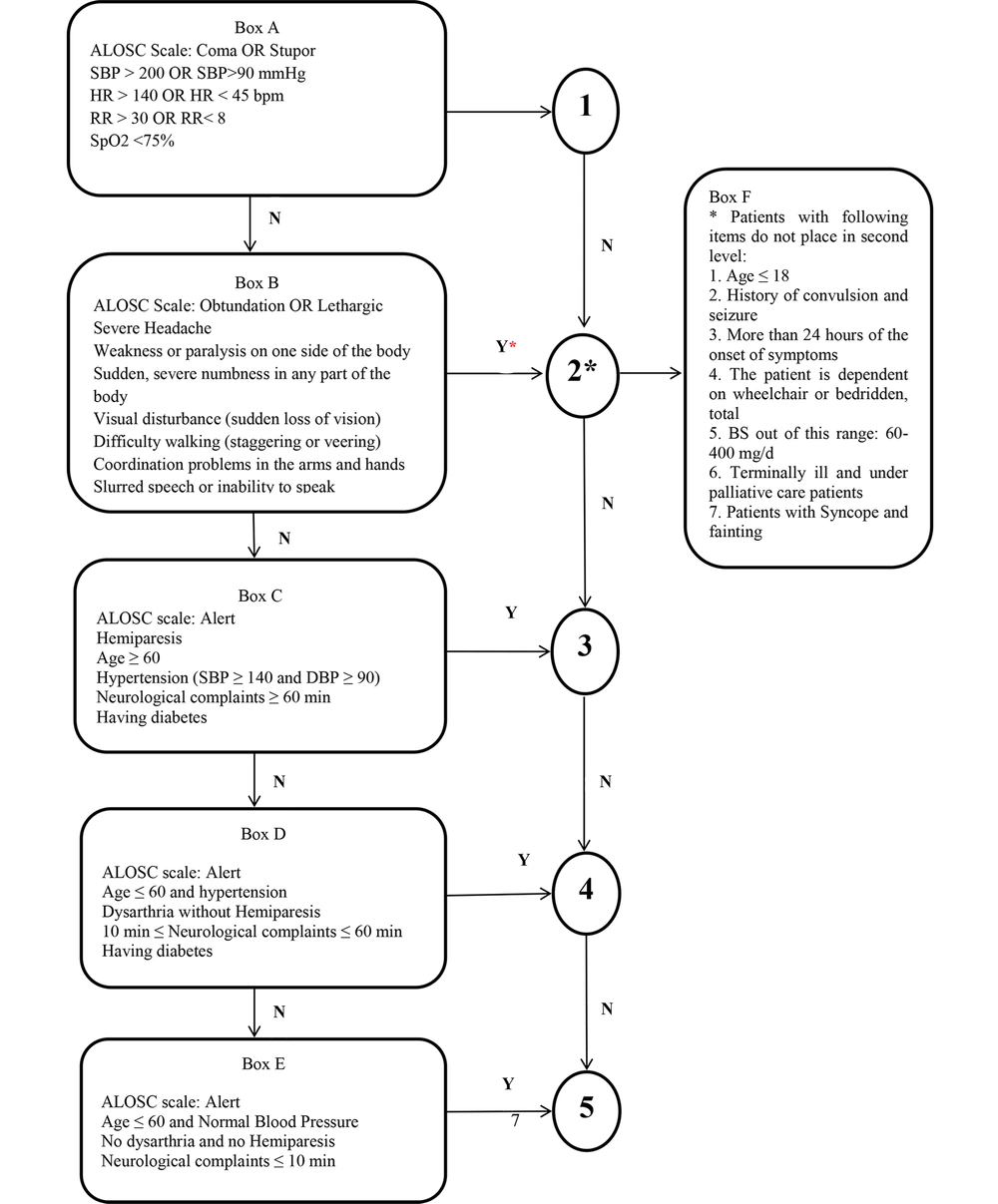
The triage of patients presenting with neurological complaints (NCs) is of utmost importance. In 2018, the authors developed a valid and reliable tool, the Stroke Triage Scale (STS) (1), to help nurses diagnose and triage cases with NCs to minimize errors and the morbidity and mortality of stroke patients. The sensitivity and specificity of STS in identifying and classifying patients with NCs, including stroke and transient ischemic attack (TIA), were 92% and 100%, respectively. Some STS items can contribute to the high stability of its sensitivity and specificity. This tool does not refer to diseases mimicking stroke symptoms. The tool has been recently modified as the Iranian STS (ISTS) (Figure 1), and some criteria have been supplemented.
The most essential item in the ISTS is seizures. Of note, the existing tools on the triage of the stroke and TIA patients, including the Los Angles Motor Scale (LAPSS), the Maria Prehospital Stroke Scale Score (MASS), the medic prehospital assessment for code stroke (MedPACS), the ontario prehospital stroke screening (OPSS), and the recognition of stroke in the emergency room (ROSIER), merely screen patients with a history of seizures and even consider them as a mimic of strokes (2, 3). Although, ischemic strokes are more common than hemorrhagic ones and post-stroke seizures highly occur after ischemic strokes, some scales, such as the face, arm, speech, time (FAST), the Cincinnati Prehospital Stroke Scale (CPSS), the rapid arterial occlusion evaluation (RACE), and the prehospital ambulance stroke test (PreHAST), do not include seizure assessment (3-7). In this regard, seizures can arise following a stroke and have immediate effects, while the impact of strokes on the power of thinking and muscle control is constant. According to the ISTS, the history of seizures during patient referrals should be assessed to distinguish strokes from seizures. If an individual has no history of seizures and is experiencing stroke-like symptoms for the first time, or if he/she had a stroke beforehand (i.e., a person with a postictal seizure following a stroke may have a high risk of a revolving stroke), he/she is placed at the second level.
Another item is hypoglycemia, which mimics a stroke. Some studies have revealed that non-screening patients with hypoglycemia can increase the number of false positives. Tools such as the PreHAST, the FAST, the RACE, the ROSIER, and the CPSS do not consider hyperglycemia as an exclusion criterion (4-7). In contrast, this criterion is considered in the OPSS (8). According to the ISTS, patients' blood sugar (BS) should be checked using a glucometer. Patients referred with NCs and BS in the 60 - 400 mg/dL range should be recognized as stroke cases and placed at the second level. If these patients have a BS beyond this range with a decreasing consciousness level, they are recognized as hypoglycemic or hyperglycemic patients.
Moreover, individuals with an incurable disease, receiving palliative care, or terminally ill patients referred with NCs, are not assigned to the second level. The OPSS also excludes these patients and those undergoing palliative care (8).
The other item is the new onset of symptoms. In this sense, the ROSIER differentiates stroke and stroke mimics by adding this item. It helps to separate new cases from old ones (6). The MedPACS also considers the new onset of symptoms (9); hence, this item in the ISTS is considered an exclusion criterion. Furthermore, patients are not placed at the second level if above 24 hours has passed since the onset of the symptoms.
Syncope is another item, which may mimic stroke and TIA. Given that hemodynamic-dependent focal neurologic (FN) deficits can occur during syncope and then be misdiagnosed as a stroke or TIA, patients are exposed to more aggressive treatments to lower their blood pressure (BP). This would aggravate syncopal episodes and cause more severe neurological sequelae. Syncope is also a transient loss of consciousness caused by global cerebral hypoperfusion with a rapid onset, short duration, and complete recovery. It is not accompanied with the FN co-occurrence (10). Accordingly, the differentiation of stroke/TIA and syncope in the ISTS is required.
Moreover, estimating the level of consciousness using the Glasgow Coma Scale (GCS) can be time-consuming as such the items of the altered level of Consciousness Scale (ALOCS; coma, stupor, obtundation, lethargy, and alertness) are included in the ISTS. Furthermore, boxes C, D, and E were also modified according to such items so that nurses can easily classify the TIA patients.
In brief, box F was added to the ISTS. If the referred patients show any of the symptoms of NCs, a cerebrovascular accident is suspected. The items of box F are checked to ensure that the person really has a stroke or that the symptoms are due to a stroke-like illness. Box F also has some exclusion criteria extracted from other scales. The exclusion criteria are age below 18 years, a history of seizures, hypoglycemia, terminally-ill patients, above 24 hours since the onset of symptoms, and syncope. If the patients are not positive in any of the aforementioned criteria, place them at the second level.
The ISTS is a practical scale for nurses in emergency departments (EDs) and prehospital settings to triage patients with NCs accurately. Like the STS, the ISTS has five levels. This five-item scale encompasses five stages. At the first stage (box A), vital signs, blood oxygen saturation, and the level of consciousness are checked using the ALOCS according to Figure 1. If patients are unstable in terms of vital signs and have severely-lost consciousness (coma or stupor), they are assigned to the first triage level and are quickly transferred to the resuscitation room. If the vital signs of patients with NCs (items identified in box B) are relatively stable with obtundation or lethargy of the ALOCS, the history of the disease is examined, and a high-risk clinical examination is performed. If patients do not meet any of the exclusion criteria in box F, they are placed at the second level. Patients are also assigned to the third (C), fourth (D), and fifth levels (E) if they do not meet the criteria in box B regarding age, gender, BP, TIA duration, diabetes, and consciousness level.
Although the 724 code is activated by emergency medical technicians (EMTs) in Iran after suspecting stroke or TIA, not all patients diagnosed and admitted to EDs have a stroke. According to the ABCD2 score for age, blood pressure, clinical features, duration of TIA, and presence of diabetes, patients with TIA may range from mild to high risk. On the other hand, some patients are referred to EDs without calling emergency medical services. In this regard, Abedi et al. reported that 74.89% of the patients receiving scores from 3 to 5 were discharged, indicating that they did not usually experience strokes during the first week, causing an optimal reduction in the ED workload (1). Taguchi et al. also mentioned the usefulness of the ABCD2 score to distinguish high-risk from low-risk cases (11).
Since the ISTS parameters are close to reality and meet the ABCD2 score criteria at the third, fourth, and fifth levels, they can differentiate low-risk and high-risk TIA patients. Nonetheless, the type and severity of work in EDs necessitate the employment of younger nurses. Accordingly, this scale presents more accurate triage in patients with stroke and TIA (12).