1. Background
Toxoplasmosis is an infectious disease caused by the parasite Toxoplasma gondii, which is an obligate intracellular parasite and belongs to the Apicomplexan branch (1-3). This apicomplexan parasite can infect large groups of warm-blooded vertebrates. T. gondii is a zoonotic disease that is of great importance to human’s and animal’s health; however, the cause of these problems is not definite and depends on factors, such as underlying diseases or the immune system (2-4).
T. gondii parasites typically occur in two ways, namely one through oocytes in the water or feces of cats and the other through tissue cysts in uncooked meat (4). A healthy individual can also transmit the parasites; nevertheless, the first two ways are usually more common (5-7). In addition, about 190,000 congenitally infected children are born with toxoplasmosis each year, and about one-third of the world’s population is exposed to chronic toxoplasmosis (8).
T. gondii has a high ability to spread throughout the host body and can even cross the blood-brain barrier using various chemical strategies (8). This parasite has several life cycles, one of which is the bradyzoite stage, in which the parasite multiplies slowly in the form of intracellular cysts in the tissues (4). It is said that this stage is observed only in domestic or wild cats because the oocyte stage is the final stage of the reproduction of this parasite and only occurs in the final host (4). Bradyzoites can remain dormant in the host’s body (4). When the immune system weakens the host, they multiply rapidly and cause indefinitely numerous problems for the host, such as tissue damage (9).
Toxoplasmosis can be transmitted vertically from a mother to the fetus during pregnancy and causes numerous problems, including growth retardation, mental retardation, eye and heart problems, many other diseases, and death (10-13). The fetus is infected with the parasite in the first trimester of pregnancy; this infection can cause miscarriage (13, 14). Toxoplasmosis is usually asymptomatic in individuals with sound immune systems (15-20).
Primary toxoplasmosis infection is asymptomatic or latent in individuals with a sound immune system (20). However, in individuals with a proper immune system, the use of corticosteroids (immune-boosting drugs), the weak immune system, or underlying diseases (eg, acquired immunodeficiency syndrome, cancer, or diabetes), T. gondii can cause devastating complications and diseases, such as fever, fatigue, sore throat, swollen lymph nodes, encephalitis, retinitis, and skin rash (12, 21, 22). Retinochoroiditis is a complication of congenital toxoplasmosis but remains latent in the body up to adolescence and then shows symptoms (23).
A combination of the two drugs, pyrimethamine, and sulfadiazine, is commonly used to treat the parasitic disease of toxoplasmosis (24, 25). Nevertheless, these drugs have side effects and ineffectiveness (26, 27). There is no vaccine to prevent this disease (28). Herbal medicine is an alternative treatment for various parasitic infections (27, 29-32). Among the possible new treatments to replace the antiparasitic drugs, sulfadiazine, and pyrimethamine, natural resources, especially medicinal plants, have a high position (25, 33-36).
D. polychaetum belongs to the Lamiaceae family (37). This plant is known in Iran as Zarrin-Giah Kermani (37). This plant has been considered since ancient times in Iran, especially in the Kerman region, due to its pleasing smell and treatment of colic (37, 38). No scientific studies have been performed on the in vivo anti-Toxoplasma activity of D. polychaetum essential oil to treat toxoplasmosis. Therefore, this study aimed to investigate the same in vivo anti-Toxoplasma activity of D. polychaetum essential oil.
2. Objectives
This study aimed to investigate the same in vivo anti-Toxoplasma activity of D. polychaetum essential oil. Moreover, this study aimed to investigate the effect of different concentrations of D. polychaetum essential oil on the survival rate of mice infected with T. gondii.
3. Methods
3.1. Identification of Plant and Identification and Preparation of Its Essential Oil
The aerial part of D. polychaetum was prepared, identified, and approved by Kerman province in August 2018, when the plant was fully flowering. For the reparation of the essential oil, the plant was powdered in the dry shade with an electric mill. Then, 100 g of the powder was transferred to a 2-liter distillation flask, and 1200 mL of deionized water was added. The essential oil was extracted for 3 hours using a Clevenger essential oil preparation machine. This process was repeated five times to prepare enough essential oil, each time with a new plant. Subsequently, the collected essential oil was then poured on the top and dehydrated with anhydrous sodium sulfate, stored in a dark closed container, away from light, and refrigerated.
3.2. Gas Chromatography-Mass Spectrometry (GC/MS) Analysis
An Agilent 7890A GC coupled with an Agilent 5975C mass detector with triple quadrupole mass analyzer and electronic ionization was used for the GC analysis of the essential oil. The gas chromatograph was prepared with an HP-5 GC capillary column (30 m × 0.25 mm; film thickness: 0.25 μm). The oven temperature started from 50ºC, held for 2 minutes, raised by 8ºC/min up to 250°C, followed by 250 - 330°C by 3ºC/min with the total run time of 58 minutes. The carrier gas was helium at a flow rate of 2 mL/min. The temperature used for the injector and the detector was 280°C. The MS parameters included ion source temperature (230°C), mass range (50 - 700), and ionization voltage (70 eV). The MSD ChemStation (version D.01.00) was used as operating software. A comparison of mass spectra and retention times with the literature data helped identify compounds.
3.3. Preparation and Maintenance of Toxoplasma gondii Strain
The T. gondii RH strain was used in the present study. This strain was prepared by the Department of Parasitology and Mycology of Isfahan University of Medical Sciences and was propagated and maintained in the laboratory through intraperitoneal passages in BALB/c mice.
3.4. Evaluation of In Vivo Anti-Toxoplasma Effects of Plant Essential Oil
In this study, 60 BALB/c mice aged 4 - 6 weeks with an average weight of 20 - 25 g were used to evaluate the in vivo anti-Toxoplasma effect of D. polychaetum essential oil. The BALB/c mice were divided into six groups of 10 cases. One group received phosphate-buffered saline as a negative control; two groups received pyrimethamine (25 mg/kg) and sulfadiazine (500 mg/kg), and three other groups received essential oil at concentrations of 50, 100, and 200 mg/kg, respectively. All stages of the study were conducted according to the ethical principles of working with laboratory animals. The animals were kept in special cages whose floors were covered with sawdust and wood chips. The floor of the shelves was changed and disinfected twice a week. All animals had the same environmental and food conditions.
The BALB/c mice were infected intraperitoneally with 2 × 104 of RH strain tachyzoites. Then, 24 hours after injecting tachyzoites into BALB/c mice, the plant’s essential oil at concentrations of 50, 100, and 200 mg/kg were injected daily into six groups of BALB/c mice. The fourth and fifth groups of mice that were considered positive control in this experiment, one of these groups received a dose of 25 mg/kg of pyrimethamine, and the other group received sulfadiazine at a dose of 500 mg/kg. In addition, one group (ie, the sixth group) without drug administration was considered a negative control. The BALB/c mice were evaluated daily for 14 days for control group survival and the number of survival days for each mouse.
3.5. Statistical Analysis
The optimal variable was the death time of the BALB/c mice. The results of survival analysis were expressed as mean ± standard deviation (SD). The Kaplan-Meier nonparametric method was used with a generalized log-rank test to evaluate the anti-Toxoplasma effect of essential oil in vivo. The data were analyzed using SPSS software (version 16), and the significance level was considered less than 0.05.
4. Results
4.1. Survival Rate of BALB/c Mice
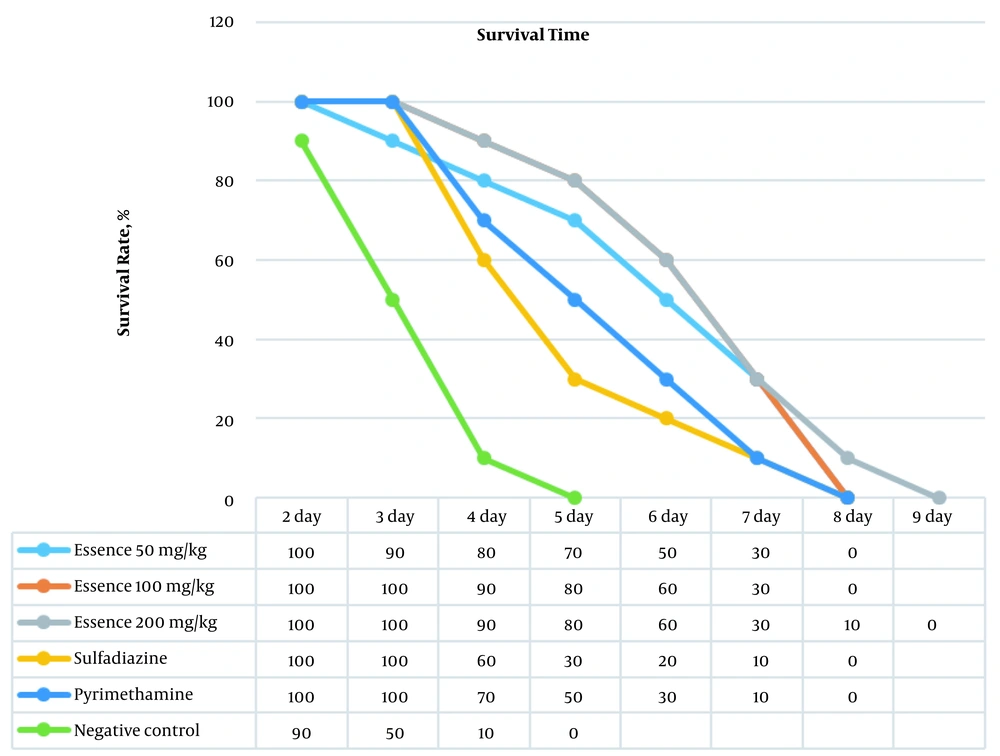
Table 1 shows the results of the generalized log-rank test for comparing the survival rate of BALB/c mice. The mean ± SD of survival rate is also shown in Table 1. There was no significant statistical difference in this regard (P > 0.05). The highest mean survival rate belonged to the concentration s of 100 (6.8 ± 0.42) and 200 (6.7 ± 0.47) mg/kg of essential oil. However, no significant difference was observed in the mean survival rate between different concentrations of the essential oil and control groups. As shown in Figure 1, the survival rate is significantly dependent on time and groups; therefore, the survival rate in the essential oil group reached 0 at a concentration of 200 on the 9th day and at concentrations of 50 and 100 on the 8th day (P < 0.05). Nonetheless, in the negative control group, it reached 0 on the fifth day.
| Groups | Mean ± SD | P-value |
|---|---|---|
| Essence (50 mg/kg) | 5.9 ± 0.68 | 0.46 |
| Essence (100 mg/kg) | 6.8 ± 0.42 | |
| Essence (200 mg/kg) | 6.7 ± 0.47 | |
| Sulfadiazine | 5.5 ± 0.50 | |
| Pyrimethamine | 5.9 ± 0.51 | |
| Negative control | 5.1 ± 6.9 |
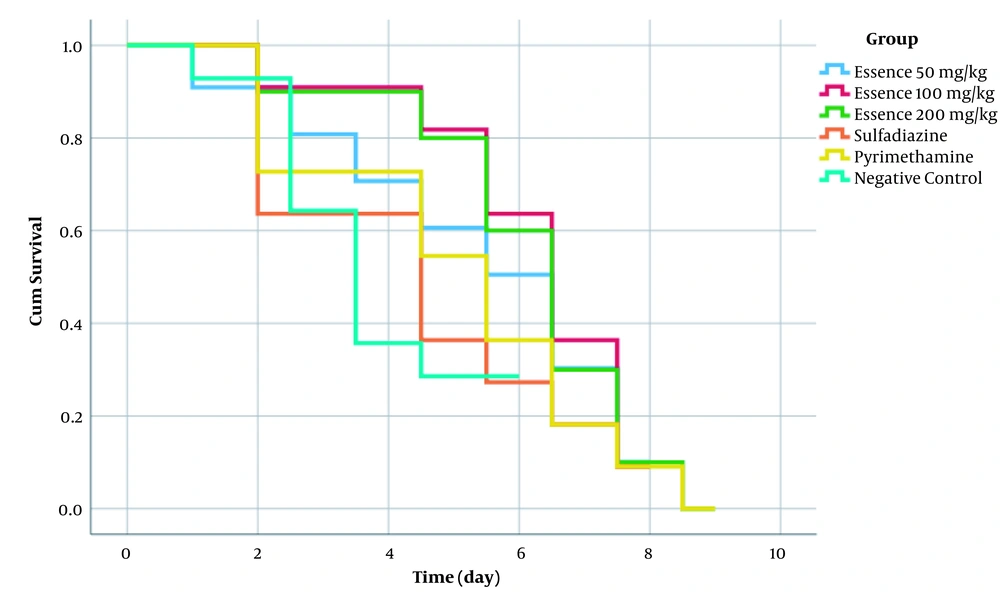
The obtained results showed that the death of each mouse led to a 10% decrease in the chart in each group. The highest survival was observed in a group of mice treated with a 200 mg/kg daily concentration of D. polychaetum essential oil (Figure 1). Figure 2 illustrates the Kaplan-Meier curve of the survival rate of BALB/c mice by groups. According to Figure 2 and the obtained results of the generalized log-rank test, it was observed that there was no significant difference between the groups.
5. Discussion
T. gondii is an intracellular parasite that can cause numerous problems for the infected individual in case of a weak immune system any underlying diseases (20, 39, 40). It can also cause severe problems in the fetus, even abortion (20). In the present study, D. polychaetum was also selected for this purpose. This plant has been helpful in traditional Iranian medicine for the treatment of numerous diseases (37, 38).
In a 2012 study carried out by Sonboli et al., the results of bioassays showed that all tested Gram-positive and Gram-negative bacteria were severely inhibited in the presence of D. polychaetum essential oil and the main studied components (41). The most susceptible microorganisms to essential oils were Staphylococcus epidermidis, with the lowest minimum inhibitory concentration (MIC) of 0.3 mg/mL (-1) (41). Pseudomonas aeruginosa was severely inhibited by Gram-negative D. polychaetum oil with a MIC of 2.4 mg/mL-1(41).
In a study conducted by Ebrahimzadeh et al. in 2017 (23) with the aim of investigating the methanolic extracts of Feijoa sellowiana, Quercus castaneifolia, and Allium paradoxum plants by the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide method, the results showed that BALB/c mice treated with the methanolic extract of Feijoa sellowiana at 200 mg/kg daily survived much better and higher than other BALB/c mice in different groups (23). This study is similar to a study performed by Khamesipour et al. in 2020 (36) that aimed to study the anti-Toxoplasma activity of D. kotschyi essential oil in vivo and in vitro (36). Moreover, BALB/c mice treated with the essential oil of D. kotschyi at a concentration of 200 mg/kg/day had a more prolonged survival than other treated groups (36).
In this study that aimed to evaluate the in vivo anti-Toxoplasma effect of D. polychaetum on BALB/c mice, the mice with a daily concentration of 200 mg/kg of D. polychaetum essential oil had longer survival rate than other groups in the experiment. The results of this study and the results of studies conducted by Ebrahimzadeh et al. and Khamesipour et al. showed that BALB/c mice treated with 200 mg/kg of essential oil or herbal extract had longer survival than other groups (23, 36). This similarity in the results of these three studies might be due to the similarity of the active compounds, such as limonene and linalool, in the herbal medicines used in the studies (36, 41, 42).
A study conducted by Mirzaalizadeh et al. in 2018 (43), investigating the effects of the methanolic extracts of Aloe vera and eucalyptus plants in vivo and in vitro, showed that BALB/c mice fed with 100 mg/kg daily concentration of the methanolic extract of eucalyptus plants were treated and had a higher survival rate than other BALB/c mice in control groups (43). The results of the study by Mirzaalizadeh et al. (43) are different from the results of studies by Ebrahimzadeh et al. (23) and Khamesipour et al. (36) and the current study. The reason could be the differences in the type of treatment that was the oral method in the study by Mirzaalizadeh et al. (43). However, in the studies by Ebrahimzadeh et al. (23) and Khamesipour et al. (36) and this study, the intraperitoneal injection was the type of treatment (23, 36, 43).
A study carried out by Leesombun et al. in 2016 (25) aiming to investigate the anti-Toxoplasma activity of Thai Piperaceae extract showed that BALB/c mice treated with Piper betle extract for 7 days after infection with the T.gondii tachyzoite strain had increased survival rates (25). The BALB/c mice receiving 400, 100, and 25 mg/kg of the plant extract had survival rates of 100%, 83.3%, and 33.3%, respectively (25).
A study conducted by Mahmoudvand et al. in 2020 (44) investigating the anti-Toxoplasma activity of Zataria multiflora essential oil showed that infected saline-treated control group of BALB/c mice had 100% mortality on the fifth day (44). However, the group that received the essential oil died entirely with a few days of delay (44).
The BALB/c mice infected with acute toxoplasmosis, which received doses of 0.2 and 0.4 mL/kg of Zataria multiflora essential oil orally, significantly increased their survival rate (P < 0.05) (44).
In a study conducted by Alnomasy in 2021 (28) with the aim of in vitro and in vivo anti-Toxoplasma effects of Allium sativum essential oil against the T. gondii RH strain, the results showed that BALB/c mice treated with Allium sativum essential oil 14 days before infection with T. gondii were treated with Allium sativum essential oil at concentrations of 200, 400, and 600 μg/kg/day for 14 days after infection (28). The mortality durations were 6, 7, and 8 days after infection, respectively. The highest survival rate in the BALB/c mice treated with Allium sativum essential oil at a concentration of 600 μg/kg/day was 3 days longer than the infected BALB/c mice in the control group (28).
In this study, the survival rate of the BALB/c mice receiving a daily concentration of 200 mg/kg of D. polychaetum essential oil was longer, which is similar to the results of studies by Leesombun et al. (25), Alnomasy (28), and Mahmoudvand et al. (44). This similarity could be due to the fact that all of the aforementioned studies used herbal medicines, and herbal medicines have a better effect on the treatment of toxoplasmosis.
5.1. Conclusions
The present study has firstly reported the in vivo anti-Toxoplasma activity of D. polychaetum. The results obtained in this study attributed the inhibitory activity of D. polychaetum essential oil against T. gondii, without toxicity, to the host. It can be assumed that the mechanism of action of the essential oil against T. gondii is associated with the mitochondrial function of T. gondii. The essential oil of D. polychaetum has an interesting pharmacological potential to be valued in the fight against toxoplasmosis. Supplementary studies are necessary to identify active compounds associated with anti-Toxoplasma activity. A concentration of 200 mg/kg of D. polychaetum essential oil had a greater significant anti-Toxoplasma effect than other groups.