1. Background
Inflammatory bowel disease (IBD) includes chronic immune system-mediated inflammatory disorders characterized by periods of remission and flare and affects the gastrointestinal tract and specially bowels with two main phenotypes, ulcerative colitis (UC) and Crohn’s disease (CD) (1, 2). The UC only involves the colon and rectum and presents with symptoms such as rectal bleeding, diarrhea, abdominal pain, and weight loss. Nonetheless, CD can affect any part of the gastrointestinal tract from the oral cavity up to the anorectal region, with the involvement of the terminal ileum and proximal part of the colon as the most prevalent subtype (3). The prevalence and incidence of IBD are increasing worldwide, and although the etiology has not been clarified yet, its main characteristics are inappropriate inflammatory responses of the bowel immune system to environmental triggers, which result in chronic tissue damage, clinical symptoms, and intestinal and/or extraintestinal complications (4, 5).
Researchers believe that the overexpression of proinflammatory mediators, such as tumor necrosis factor-alpha (TNF-α), has the main role in the pathogenesis of IBD (6-8). Despite significant progress in the management and control of disease activity, there is no definite curative approach for these disorders (8, 9). During the last decades, the achievement of biological medicines, especially those that target TNF-α, has revolutionized the management of IBD (1, 10). One of the anti-TNF medications, which has shown to be effective for the induction and maintenance of IBD remission, especially in moderate to severe unresponsive cases, is adalimumab (7, 11). Adalimumab binds to TNF-α, and by inhibiting its activity not only suppresses the symptoms of IBD but also induces remission as the silent mode of disease. Furthermore, adalimumab can keep the condition under control for the long term (12, 13). Despite therapeutic efficacy, there are side effects, such as susceptibility to infections and hypersensitivity reactions, which sometimes limit the clinical application of anti-TNFs (6, 9). Although adalimumab is approved for the management of IBD, especially in refractory cases, the evidence regarding its long-term efficacy and safety is limited, and most of the investigations have been performed on subjects who experienced previous treatment with anti-TNF family (3, 8, 14, 15).
2. Objectives
The current study was designed to evaluate the efficacy of adalimumab biosimilar (CinnoRa, CinnaGen Co., Tehran, Iran) for the management of IBD in the southwest region of Iran. Moreover, this study evaluated any potential side effects or related complications of this medicine.
3. Methods
During this prospective observational study, which was defined and designed for one year, the patients with active IBD who were unresponsive to previous medications and referred to the IBD outpatient clinic of Ahvaz Imam hospital as a tertiary center, Ahvaz, Iran, were included. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki; therefore, this study was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.HGOLESTAN.REC.1399.076). Before participation, the method of study was explained to all the participants, and they were requested to sign a consent form. They were in touch with a clinician by phone call and were requested to report any potential complications or side effects.
The inclusion criteria were subjects with active IBD, the age range of 18 - 80 years, and unresponsive or intolerant to previous medications, including immunomodulators, such as azathioprine. The exclusion criteria were a history of malignancy, severe heart failure, active tuberculosis, human immunodeficiency virus or infection, acute or severe hepatitis B or C, and history of therapy with adalimumab for the management of other immune-mediated disorders, such as rheumatoid arthritis or psoriasis.
At the beginning of the study, the basic characteristics of participants, including the type of IBD, age, gender, any concomitant disease, and smoking and drug history, were recorded. The informative data regarding IBD, including the type and location of disease, age at the time of diagnosis, the time lag between disease presentation and diagnosis, duration of involvement with IBD, and intestinal and/or extraintestinal symptoms, were extracted from patients’ files. The patients were scheduled to receive adalimumab (160, 80, and then 40 mg SC QOW (subcutaneously once every other week)) and be followed for 52 weeks.
3.1. Evaluation of Treatment Efficacy
Disease activity was defined based on the Mayo score for UC cases (maximum score 12, clinical remission ≤ 2, and clinical response ≥ 2 score decrease) and the Crohn’s disease activity index (CDAI) for CD cases (clinical remission < 150, mild to moderate activity: 150 - 220, moderate to severe activity: 221 - 450, and 451 - 1100 as fulminant and very severe flare) (16). Primary treatment failure was defined as no improvement in clinical symptoms after a loading dose (first injection: 160 mg), and secondary treatment failure was defined as the primary response followed by loss of response or intolerance of treatment due to side effects or need for colectomy.
The efficacy of treatment, including clinical symptoms, disease activity index score, injection-related side effects, rate of clinical and endoscopic remission or flare, duration of remission, rate of treatment failure, colectomy, any severe infection which mandates holding treatment, need for hospital admission, and any mortality during the 0, 12th, 24th, and 52nd weeks, was investigated and recorded.
3.2. Statistical Analysis
The SPSS software (version 22.0) was used for data analysis. The data are expressed as mean, standard deviation, and percentage. The data were checked for normality using the Kolmogorov-Smirnov and Shapiro-Wilk tests. The Mann-Whitney U test and independent two-sample t-test were employed to compare two quantitative variables. The chi-square test was used to evaluate the association between two qualitative variables. A P-value less than 0.05 was considered statistically significant.
4. Results
Overall, a total of 71 patients, including 42 male and 29 female, with a mean age of 29 years, were included in this study. Table 1 shows the demographic characteristics of the participants. In this study, 37 and 34 patients were diagnosed with UC (52.1%, 20 male and 17 female) and CD (47.8%, 22 male and 12 female), respectively. The time to remission in the UC group was significantly longer than in the CD group (10.05 and 1.71 months; P < 0.0001). After treatment with adalimumab, the clinical remission rate (≥ 2 points reduction in the Mayo score) in the 12th week among UC patients was 67.5% (n = 25) and raised to 100% (all the UC patients) in the 24th and 52nd weeks. None of the UC patients experienced disease recurrence.
| Variables | UC (n = 37) | CD (n = 34) | P - Value |
|---|---|---|---|
| Average age (range) | 30.8 (19 - 47) | 35.2 (23 - 47) | 0.012 |
| Gender | 0.47 | ||
| Male | 20 | 22 | |
| Female | 17 | 12 | |
| Smoking | 3 (8.1) | 5 (14.7) | 0.467 |
| Symptom to diagnosis lag (range, mo) | 4.08 ± 2.19 (1 - 9) | 4.44 ± 2.27 (2 - 10) | 0.49 |
| Duration of involvement (mo) | 4.73 ± 2.13 (2 - 10) | 4.84 ± 2.18 (2 - 9) | 0.889 |
| Drug history | |||
| Sulfasalazine | 17 (45.9) | 14 (41.2) | 0.811 |
| Prednisolone | 11 (29.7) | 13 (38.2) | 0.465 |
| Azathioprine | 36 (97.3) | 34 (100) | 1.000 |
| 5-aminosalicylic acid | 2 (5.4) | 0 | 1.000 |
| Anatomical location | |||
| Small bowel | 0 | 10 (29.4) | 0.0001 |
| Large bowel | 15 (40.5) | 3 (8.8) | 0.003 |
| Small and large bowels | 22 (59.5) b | 21 (61.8) | 1.000 |
| Complication | |||
| Anal fistula | 0 | 5 (14.7) | 0.021 |
| Abscess | 0 | 2 (5.9) | 0.226 |
| Intraabdominal fistula | 0 | 1 (2.9) | 0.479 |
Demographic Characteristics of Participants a
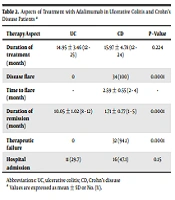
In CD patients, the CDAI significantly decreased during the treatment time; nevertheless, all CD patients (100%) experienced disease recurrence after a mean time of 2.59 ± 0.55 months (within 2 - 4 months) after treatment with adalimumab (P < 0.0001) (Table 2). Failure of treatment was observed in 94.1% of CD patients (n = 32); however, none of the UC patients had treatment failure (P < 0.0001). There were no complications related to adalimumab, and no patients needed colectomy during the study period. Table 3 shows the changes in disease activity scores during the study indicating a significant decrease during the course of the study (P < 0.001).
| Therapy Aspect | UC | CD | P - Value |
|---|---|---|---|
| Duration of treatment (mo) | 14.95 ± 3.46 (12 - 25) | 15.97 ± 4.78 (12 - 24) | 0.224 |
| Disease flare | 0 | 34 (100) | 0.0001 |
| Time to flare (mo) | - | 2.59 ± 0.55 (2 - 4) | - |
| Duration of remission (mo) | 10.05 ± 1.02 (8 - 12) | 1.71 ± 0.77 (1 - 5) | 0.0001 |
| Therapeutic failure | 0 | 32 (94.1) | 0.0001 |
| Hospital admission | 11 (29.7) | 16 (47.1) | 0.15 |
Aspects of Treatment with Adalimumab in Ulcerative Colitis and Crohn’s Disease Patients a
| Activity Score (Average) | UC (Mayo Score) | CD (CDAI) |
|---|---|---|
| Beginning of therapy | 11.32 ± 0.47 (11 - 12) | 160.77 ± 4.77 (155 - 168) |
| 12th week | 9.35 ± 1.23 (9 - 11) | 149.26 ± 3.17 (145 - 162) |
| 24th week | 4.89 ± 2.45 (3 - 8) | 146.42 ± 4.15 (140 - 155) |
| 52nd week | 4.11 ± 1.47 (3 - 6) | 137.03 ± 8.38 (125 - 155) |
| P - value | 0.0001 | 0.001 |
Disease Activity Scores During Study
5. Discussion
During the current study, the clinical response (2 or more score decrease in the Mayo score) was achieved in 67.5% of UC cases in the 12th week and raised to 100% (all cases) up to the 24th and 52nd weeks. None of them experienced treatment failure or flare. The aforementioned results proved the high rate of adalimumab efficacy for the management of refractory UC. In a similar investigation, Ogata et al. evaluated adalimumab efficacy in a multicenter study and reported clinical remission of 49.7% in the 4th week and 74.4% in the 52nd week based on the Mayo score (17). Other studies have also certified that adalimumab can control most UC cases as soon as the 8th week of therapy and maintain this response up to the 52nd week (18, 19). Angelison et al.’s study conducted on 118 UC patients with adalimumab for 3 months achieved clinical remission among 77% of participants, and the response rate of those with a history of treatment with infliximab was lower than naive patients (73% and 85%), which is in line with the results of the current study (20). Another study by Balint et al. investigated adalimumab efficacy on 73 refractory UC cases (15). Based on the Mayo score, in the 12th and 52nd weeks, clinical remission was observed in 75.3% and 92% of participants, respectively (15). Travis et al.’s study on 436 moderate to severe UC patients showed that up to the 26th week, 67% of the cases would respond to treatment, and based on the simple clinical colitis activity index, 48% of the cases were in remission (21).
In Iborra et al.’s study, the rates of clinical remission of UC cases after 1 year among naive and nonnaive patients were 65% and 49%, respectively (1); nevertheless, in the current study, the rate of clinical remission was 100%. This difference can be explained based on the differences in patients, duration of disease involvement, previous medications, and rate of compliance. Ultra 1 and 2 studies have proved the efficacy of adalimumab for the management of UC as the first randomized controlled trials (22, 23). Despite this proven efficacy, the results of the current study are more efficient than previous studies.
During the current study, all the CD cases (100%) experienced disease flare 2 to 4 months after starting the therapy with adalimumab, and 94.1% of them (n = 32) demonstrated treatment failure. The inefficacy of anti-TNFs during the first year of therapy could be due to special disease characteristics that TNF-α is not the main proinflammatory cytokine in pathogenesis, and other metabolic pathways induce inflammation (10). A cohort study by Bouhnik et al. evaluating adalimumab efficacy for the management of CD patients and symptomatic small bowel stricture showed that 64% of patients were successfully treated up to the 24th week, and 45.7% of them maintained this remission up to the 4th year of follow-up (4). In this study, successful treatment based on clinical symptoms and imaging findings was defined as the steroid-free continuation of management with adalimumab after 8 weeks and no need for other anti-TNFs, endoscopic dilation, or bowel resection (3). This result contradicts the findings of the current study and could explain the differences in characteristics of patients and evaluation of outcomes.
Loftus et al. evaluated the efficacy of adalimumab for the management of 2057 moderate to severe naive CD patients in 6 years (24). Based on their results, the rate of clinical remission (Harvey-Bradshaw index < 5) from 29% at the beginning increased to 68% in the first year up to 75% in the 6th year. Moreover, the patients with a history of under 2 years of involvement achieved a higher rate of remission (24). Loftus et al. concluded that routine management of CD with adalimumab for up to 6 years could improve disease outcomes and rate of clinical remission, and there is no concern about drug safety (24). The aforementioned results are also inconsistent with the results of the current study. One explanation for the variation of results could be the difference in the evaluation of clinical remission. In the current study, although the duration of involvement for all the participants was more than 2 years, it has been mentioned in previous studies that the early treatment of CD with anti-TNF (less than 2 years since diagnosis) could result in more efficacy (25-27).
During the current survey, none of the participants had adalimumab-related side effects, which proved the safety of this drug among IBD patients. However, about one-third of UC cases and one-half of CD cases need hospital admission due to various reasons in the first year of therapy with adalimumab, and this issue increases the direct and indirect costs of these disorders (28). This hospital admission rate is in line with that of Iborra et al.’s study (1). On the other hand, the need for colectomy was not observed in the participants; nonetheless, most observational studies have reported the colectomy rate within the range of 23 - 46% (29, 30). Additionally, in Balint et al.’s study, 5.4% of moderate to severe UC cases required colectomy during the first year of therapy with adalimumab (15). This discrepancy could be due to the exclusion of hospitalized patients from the present study at the beginning.
Overall, the safety profile of adalimumab is in line with those of other studies, such as McDermott et al.’s survey (8). Colombel et al. also reported the high tolerance rate and safety profile of adalimumab during a 56-week observation (31). Based on different studies, the most common side effects related to IBD treatment with adalimumab include infections (11 - 34%), malignancies (1.9%), and demyelinating disorders (0.7%) (17, 20, 24, 31). The report of no side effects in the current study proved that there is no new concern about the adalimumab safety profile. Furthermore, based on the high efficacy rate among UC patients, adalimumab could be an ideal choice for refractory cases.
One of the limitations of the current study is evaluating and following therapeutic results only for one year. On the other hand, this study was performed as a real-life treatment with the possibility of concomitant consumption of other medications, such as corticosteroids or immunomodulators, which can affect the achievement of a better outcome. Moreover, this study was carried out as a single-center survey with a small number of cases.
5.1. Conclusions
Adalimumab has a positive effect on the improvement of clinical symptoms, reduction of disease activity, prevention of disease recurrence, and need for colectomy in moderate to severe UC patients. However, adalimumab has no efficacy in the improvement of CD patients, and failure of treatment was observed in most of these patients. Adalimumab could be a therapeutic option for the management of UC with prior failure of treatment.