1. Background
Toxocariasis is a common zoonotic helminthic disease primarily distributed in subtropical and tropical regions (1). Humans can be infected with toxocariasis by ingesting soil and vegetables contaminated with the eggs of Toxocara canis or Toxocara cati. In some cases, the infection can be acquired by direct contact of hands with the hair of dogs or, in rare cases, via the consumption of larvae in the raw meat of Toxocara-infected paratenic hosts.
Humans are accidental hosts for toxocariasis. The parasite can complete its life cycle in humans. Therefore, eggs are not excreted in human fecal samples (2). Human toxocariasis, in most cases, is clinically asymptomatic. Clinical manifestations of toxocariasis include visceral larva migrans (VLM), ocular larva migrans (OLM), and covert and atypical toxocariasis according to the affected organ (3). The diagnosis of human toxocariasis is performed mainly by a combination of clinical examination and enzyme-linked immunosorbent assay (ELISA) for the excretion-secretion antigen (4).
Seroprevalence varies from as low as 1% in Spain to 86% in Santa Lucia, with a tropical climate (5). Based on seroprevalence studies, toxocariasis is more prevalent in socioeconomically poor children in tropical and subtropical regions. The seroprevalence of human toxocariasis is predominant in people under 20 years and urban regions in different parts of Iran. The prevalence of human toxocariasis with overall 28 records from all over Iran was reported as 9.3% (95% CI: 6.3 - 13.1%) (6).
Several risk factors are related to the prevalence of Toxocara infection in humans, such as keeping cats and dogs and raw consumption of vegetables (7).
2. Objectives
However, sero-epidemiological data related to human toxocariasis and its risk factors are limited in eastern Iran. Therefore, we studied the seroprevalence of human toxocariasis and associated risk factors among clinically healthy individuals in Birjand district, eastern Iran, for the first time. This study aimed to attract public attention to Toxocara infection in the country’s eastern regions, especially South Khorasan province.
3. Methods
3.1. Study Location and Patient Selection
A cross-sectional study was performed in Birjand, Iran. South Khorasan province is located in the east part of Iran, on the border with Afghanistan, with a predominant desert climate (8). According to a 25% infection prevalence in a previous study (9), the sample size was calculated as follows:
In order to adjust for missing data, the sample size was increased to 450 people selected from clinically healthy individuals between March and May 2022. The healthy subjects above 18 years old who were voluntarily admitted to hospitals for health screening were regarded as clinically healthy individuals. The exclusion criteria were hemolyzed samples and infection with other intestinal parasites.
3.2. Data Collection
A questionnaire was used to collect risk factors and epidemiological data (age, gender, residential area, washing hands before eating, consuming raw/undercooked meat and vegetables, and contacting cats and dogs). The content validity of this questionnaire was checked and confirmed by experts. The reliability of the questionnaire was also confirmed owing to the questions’ objectiveness and frequent use in previous studies.
3.3. Sampling and Laboratory Analysis
Approximately 5 mL of blood was collected from each participant in a clot activator tube. The collected sera were stored at -20°C until testing. The IgG antibodies against Toxocara were detected by T. canis excretory-secretory (TES) antigens using a commercial ELISA kit (IBL, manufactured in Hamburg, Germany) following the manufacturer’s instructions. Briefly, serum samples were incubated and conjugated with alkaline phosphatase, followed by the tetramethylbenzidine (TMB) substrate.
Absorbance readings were performed at 450 nm. The average absorbance reading for three negative control sera plus two standard deviations was considered a cutoff value. The OD values ≥ 0.3 were reported positive.
3.4. Statistical Analysis
Frequency analysis was performed to describe the participants’ characteristics and the parasite prevalence. Logistic regression was used to analyze the association between toxocariasis and potential risk factors by SPSS 21.0 software. Odds ratios (ORs) were considered statistically significant if the 95% CI did not include one. A probability P value less than 0.05 was considered statistically significant.
4. Results
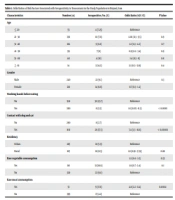
A total of 450 clinically healthy individuals (210 men and 240 women) aged 18 to 81 years (mean 35 ± 13 years) were randomly referred to medical laboratories for health screening in Birjand, Iran. Table 1 shows the demographic characteristics of the sample. The majority (28.8%) of individuals were in the age range of 21 - 30 years. The Toxocara ELISA was positive in 36 (8%) of the 450 individuals. The highest prevalence rate of toxocariasis was reported in individuals over 61 years old (14.2%, 95% CI 0.3 - 12.9) (Table 1). Moreover, statistical analysis showed that washing hands before eating (OR = 0.1, 95% CI 0.05 - 0.3), P < 0.0001), contact with cats and dogs (OR = 0.1, 95% CI 0.05 - 0.3, P < 0.00001), and raw meat consumption (2.2 - 11.4) (OR = 4.8, 95% CI 2.2 - 11.4), P < 0.0004) were risk factors associated with Toxocara infection. However, there were no relationships between toxocariasis and age, gender, residency, and raw vegetable intake.
| Characteristics | Number (n) | Seropositive, No. (%) | Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Age | ||||
| ≤ 20 | 55 | 4 (7.25) | Reference | |
| 21 - 30 | 130 | 10 (7.6) | 1.06 (0.3 - 3.5) | 0.9 |
| 31 - 40 | 102 | 9 (8.8) | 1.2 (0.3 - 4.2) | 0.7 |
| 41 - 50 | 99 | 7 (6) | 0.9 (0.2 - 3.4) | 0.9 |
| 51 - 60 | 40 | 4 (10) | 1.4 (0.3 - 6) | 0.6 |
| ≥ 61 | 14 | 2 (14.2) | 2.1 (0.3 - 12.9) | 0.4 |
| Gender | ||||
| Male | 240 | 22 (9.1) | Reference | 0.3 |
| Female | 210 | 14 (6.6) | 0.7 (0.3 - 1.4) | |
| Washing hands before eating | ||||
| No | 190 | 30 (15.7) | Reference | |
| Yes | 260 | 6 (2.3) | 0.1 (0.05 - 0.3) | < 0.0001 |
| Contact with dog and cat | ||||
| No | 290 | 8 (2.7) | Reference | |
| Yes | 160 | 28 (17.5) | 7.4 (3.3 - 16.8) | < 0.00001 |
| Residency | ||||
| Urban | 285 | 18 (5.9) | Reference | |
| Rural | 165 | 18 (11.5) | 1.8 (0.91 - 3.59) | 0.08 |
| Raw vegetable consumption | 1.2 (0.6 - 2.5) | 0.53 | ||
| Yes | 110 | 12 (10.9) | 1.6 (0.7 - 3.4) | 0.1 |
| No | 320 | 22 (6.8) | Reference | |
| Raw meat consumption | ||||
| Yes | 51 | 9 (17.6) | 4.8 (2.2 - 11.4) | 0.0004 |
| No | 399 | 17 (4.2) | Reference |
5. Discussion
The current study evaluated the seroprevalence of toxocariasis among clinically healthy individuals and potential factors associated with Toxocara infection in South Khorasan province, eastern Iran. The prevalence of toxocariasis among asymptomatic individuals worldwide varies from 2% to 80% (10). Many factors, such as lifestyle, geographical conditions, and detection methods, play a role in such differences. Moreover, the seroprevalence of anti-Toxocara IgG antibodies was significantly lower in the present study than in previous studies in Peru (44.92%) (11), United States (13.9%) (12), Brazil (51.6%) (13), and South Korea (51.2%) (14). The prevalence of anti-Toxocara IgG was higher in the current study than in the same study from Denmark (2.4%) (15) and approximately consistent with an Egyptian study (7.7%) (16). In this study, the overall seroprevalence of IgG antibodies against toxocariasis was 8%, slightly lower than the mean value (9%) of previous studies conducted in different parts of Iran (6, 17). In a previous study performed in Iran, 49 (15.54 %) healthy people were seropositive for toxocariasis, and a significant risk factor was contact with cats and dogs but not age and gender (18), while in the current study, the seroprevalence was 8% and a significant risk factor was washing hands before eating, contact with cats and dogs, and raw meat consumption. In the current study, seroprevalence increased along with age but was not associated with gender, residency, or raw vegetable intake. In the present study, the seroprevalence was increased with age, consistent with those reported previously (12, 14). In this study, there was no statistically significant association between toxocariasis and age, gender, residency, and raw vegetable intake.
We observed no statistically significant association between Toxocara infection seropositivity and age, gender, residency, and raw vegetable intake. However, in contrast to some studies (19, 20), we found that contact with a dog or cat could be a risk factor for Toxocariasis. In the present study, the prevalence was higher in men than women, but this difference was not statistically significant. To our knowledge, the past epidemiological studies regarding sero-epidemiological data related to human toxocariasis and its risk factors are limited in the east of Iran.
5.1. Conclusions
This study’s relatively low seroprevalence of toxocariasis can be due to environmental conditions and relatively good health status in Birjand. It is suggested that more extensive studies be conducted with larger sample sizes in at-risk groups in this area.