1. Background
Diabetes is responsible for the death of countless people across the world each year (1). According to a surprising report by Saeedi et al., almost 500 million people suffer from diabetes worldwide, and the number is expected to increase to 578 million by 2030 and 700 million by 2045 (2). Type 2 diabetes (T2D) accounts for 90 - 95% of this population, inducing various serious long-term complications, both microvascular and macrovascular (3, 4).
Chronic kidney disease (CKD) is the most prevalent diabetes-related complication (up to one in three adults with newly diagnosed T2D have CKD), which mostly develops into diabetic nephropathy (DN) after several years. DN refers to a characteristic set of structural and functional kidney abnormalities, defined as persistent proteinuria (more than 500 mg of protein or 300 mg of albumin per 24 hours) in patients without urinary tract infection or other diseases causing the proteinuria (5). According to a report by the International Diabetes Federation (IDF), DN accounts for 44% of new cases of end-stage renal disease (ESRD) and 18% of death in diabetics and is the main contributor to the total cost of diabetes care worldwide (6).
The current standard clinical methods for detecting early-stage kidney disease rely upon the assessment of kidney function, usually by calculating the estimated glomerular filtration rate (eGFR), and the assessment of kidney damage, usually by checking urinary albumin-to-creatinine ratio (ACR) (7, 8). However, ACR and eGFR have intraindividual differences and may have prognostic errors. As such, it is necessary to use alternative biomarkers to correctly identify the increased risk of diabetic kidney disease (DKD) (7, 9-12). Insulin-like growth factor-binding protein 3 (IGFBP-3) is one of the most important protein biomarkers that can predict future renal decline differently from other clinical risk factors in diabetic patients (13, 14). The involvement of IGFBP-3 in diabetic microalbuminuria and DN has been widely studied (13). The plasma level of IGFBP-3 can be associated with eGFR in patients with DN. It is also significantly higher in patients with DN than those with T2D with normal kidney function (7, 8, 15-17). Such data support the importance of IGFBP-3 in detecting the development of DN.
Nevertheless, in addition to early detection, proper intervention is also vitally necessary to control further deterioration in the kidney function of diabetic patients. Numerous studies have shown that physical activity and exercise, as a non-pharmacological treatment for a healthy lifestyle, are significantly effective in DN improvement and slowing its progression in T2D patients (18, 19). Physical activity is associated with increased eGFR, decreased urinary albumin creatinine ratio (UACR), and decreased rate of microalbuminuria in diabetics (18, 19). Nevertheless, the type of exercise is highly important. In line with this, previous studies have indicated that vigorous exercises, such as RT are more effective in improving glycemic profiles of diabetic patients than moderate training (aerobic) (20-22). RT can improve blood lipid profiles, insulin sensitivity, glucose tolerance, glycemic control, and resting blood pressure. All these factors are the major determinant mechanisms of T2D (23). Besides, since the muscular system accounts for 35 to 40% of body weight and approximately 75% of whole-body insulin-stimulated glucose uptake (24), RT, which is much more involved with the muscular system, may delay or ameliorate the development of T2D complications, such as nephropathy (3, 19, 20, 23).
Despite the logic behind RT in managing T2D, the probable effects of such an exercise on kidney function are still not clear.
2. Objectives
This study was done to investigate the impacts of an 8-week RT on the kidney function of T2D patients with the risk of DN by measuring IGFBP-3, eGFR, and glycemic blood control.
3. Methods
3.1. Subjects
Based on a simple random sampling method, 30 men with T2D (> 27 kg/m2 > body mass index (BMI) < 40 kg/m2) from the list of diabetic patients of Shiraz University of Medical Sciences were selected as the subjects of this study in June 2019 (25). This research was carried out according to the ethical principles of the World Medical Association (Helsinki Declaration) and was approved by the ethics committee of the Islamic Azad University, Marvdasht Branch, Iran (162384503). Inclusion criteria were as follows: established T2D (fasting blood sugar ≥ 126 mg/dL and 2-hour post-prandial blood glucose ≥ 200 mg/dL) and CKD stages 2 - 4 (eGFR 20 - 90 mL/min/ 1.73 m2) for more than five years, having an inactive lifestyle, male gender, and the age of 35 to 60 years. Each subject completed a previous RT experience questionnaire, which indicated that all of them had no previous experience of RT, at least for a year. Eligible subjects went under a physical examination and medical screening, and they were excluded if they had a condition that could potentially endanger their health during the study (acute renal failure and dialysis, urinary tract infection, insulin treatment, congestive heart failure, hypertension, diabetic microvascular complications, and physical problems). Before participation, subjects were informed of the possible risks of the tests and signed the informed consent.
3.2. Experimental Approach to the Problem
The current study was a quasi-experimental randomized control trial with a parallel design and a 1: 1 allocation ratio. To increase the homogeneity of sampling, based on the fasting blood sugar of the subjects, they were randomly (drawing lots) assigned to the control group (CG, n = 12) or resistance training group (RTG, n = 10) (Table 1). Five subjects from the RTG and three subjects from the CG withdrew from the study during the eight weeks because they were unable to participate in the post-tests. Therefore, out of 30 cases at baseline, just 22 subjects successfully completed the training period and tests. The subjects were unaware of which group they would be allocated to. The groups were similar at baseline regarding the anthropometric and body composition characteristics. All the measurements were taken based on the blinded method, and the laboratories' technicians were completely unaware of which group the subject had been allocated to. To avoid the probable effects of the practical test (1 RM) on acute physiological responses, the measurements related to blood samples were taken 48 hours before the practical test. The baseline laboratory measurements were taken at the Academic Center for Education, Culture, and Research (ACECR) and the Shiraz University of Medical Science, and the training sessions were carried out in a sports center in Shiraz, Iran. All the tests were repeated based on the pre-test procedure after the eighth week. All the research variables were measured based on pre- and post-test design. The subjects were asked not to change their diet until the end of the research.
| Variables | Baseline | After Intervention | P-Value Paired t-test | P-Value (ANCOVA) |
|---|---|---|---|---|
| Body mass (kg) | 0.9 | |||
| RTG | 80.1 ± 8.5 | 80.0 ± 8.0 | 0.5 | |
| CG | 78.5 ± 6.3 | 79.1 ± 5.4 | 0.1 | |
| Body mass index (BMI) (kg/m2) | 0.03 c | |||
| RTG | 27.3 ± 4.2 | 26.9 ± 3.8 | 0.04 b | |
| CG | 27.8 ± 2.3 | 28.0 ± 1.2 | 0.1 | |
| Body fat (%) | 0.001 c | |||
| RTG | 21.2 ± 3.5 | 19.6 ± 2.1 | 0.01 b | |
| CG | 22.4 ± 3.1 | 22.9 ± 2.8 | 0.1 | |
| Waist-to-hip ratio (WHR) | 0.02 c | |||
| RTG | 0.97 ± 0.04 | 0.95 ± 0.04 | 0.01 b | |
| CG | 0.95 ± 0.02 | 0.96 ± 0.04 | 0.09 |
a Values are expressed as mean ± standard deviation.
b Significant differences within group (P < 0.05).
c Significant differences between groups (P < 0.05).
3.3. Resistance Training Protocol
The training protocol involved three sessions of circuit RT program per week (three sets × 12 - 15 repetitions with 50 - 65% 1RM over the first four weeks and three sets × 8 - 10 repetitions with 65 - 80% 1RM over the second four weeks). In each training session, after a 10-minute warm-up (running on the treadmill), the subjects performed six different exercises to stress the major muscle groups (chest press, leg extension, shoulder press, leg curls, latissimus pull-down, and leg press) for 45 minutes followed by a 5-minute cool-down at the end. In an orientation meeting, all the subjects were familiarized with the instruction of the RT machines used in the training program. The intensity (load) of the exercise was determined based on the subjects’ 1RM record. 1RM was estimated from 1 – 3 RM effort (26).
3.4. Anthropometry and Body Compositions
The subjects' weight and height were measured to calculate BMI. The waist-to-hip ratio (WHR) was also calculated by dividing waist circumference (the narrowest part of the torso, above the umbilicus) by hip circumference (the maximum part of the hip while standing with the heels together). Skinfold thickness in the chest, abdomen, and thigh was measured using a skinfold caliper (Harpenden, HSK-BI, British Indicators, West Sussex, UK) and a standard technique by the same investigator to evaluate body fat percentage (Table 1).
3.5. Blood Sampling and Biochemical Analysis
Following an overnight fast, basal blood samples were drawn from an antecubital vein before the first training session and 48 hours after the last training session (Figure 1). Then, they were kept in ethylenediaminetetraacetic acid (EDTA) tubes and centrifuged at 3000 rpm for 15 minutes to remove the blood plasma for further measurement of the plasma level of IGFBP-3, creatinine, glucose, and insulin. The oxidase method was used within 12 h of blood collection to measure fasting glucose levels (kit: Pars Azmon, Iran). A fasting level of insulin and plasma level of IGFBP-3 were measured by enzyme-linked immunosorbent assay (ELISA) method using especial kits (insulin: Mercodia-Sweden with the sensitivity of 1 mg/dL and inter-assay coefficients of variation were < 1.3%, IGFBP-3: ZellBio-German sensitivity of 0.1 mg/mL and inter-assay coefficients of variation were < 10%).
3.6. Variables’ Measurement
Estimated glomerular filtration rate (eGFR) and insulin resistance index levels were calculated by the following formula, respectively (27, 28):
3.7. Statistical Analysis
All measured parameters were expressed as mean ± standard deviation (SD). To analyze and compare the results, paired t-test, covariate (ANCOVA) analysis, and effects size (ES) were used. Effect sizes of 0.2, 0.5, and 0.8 were considered small, medium, and large, respectively. The results of baseline measurements were selected as the covariate factor for further analysis. Statistical significance was accepted at P ≤ 0.05, and statistical analyses were performed by SPSS software version 26.
4. Results
Anthropometric and body composition characteristics of the subjects before and after training are presented in Table 1. No significant differences were observed in the anthropometric and body composition parameters of the subjects at baseline. As shown in Table 1, BMI, body fat percentage, and WHR decreased significantly after eight weeks of RT (P < 0.05).
4.1. IGFBP-3
Plasma levels of IGFBP-3 significantly increased (from 2.3 to 3.4 mg/mL) in the CG (F = 3.5, P = 0.05, ES: 0.19), while the plasma levels of IGFBP-3 showed no significant change (from 2.07 to 2.5 mg/mL) in the RTG after the training period. Figure 2 presents IGFBP-3 levels at pre-and post-test.
4.2. eGFR
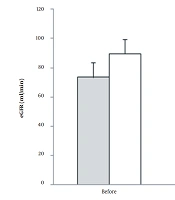
eGFR value significantly decreased (from 89.5 to 74.5 mL/min) in the CG (F = 7.4, P = 0.01, ES: 0.11), while eGFR levels approximately remained unchanged (73.4 to 74.8 mL/min) in the RTG after the training period. Figure 3 presents eGFR levels at pre-and post-test.
4.3. Fasting Blood Glucose
Fasting blood glucose levels significantly decreased (from 149.7 to 124.7) in the RTG (F = 3.7 and P = 0.05, ES: 0.4), while its levels increased (from 143.9 to 213.2) in the CG but not significantly after the training period. Figure 4 presents fasting blood glucose levels at pre-and post-test.
4.4. Fasting Insulin
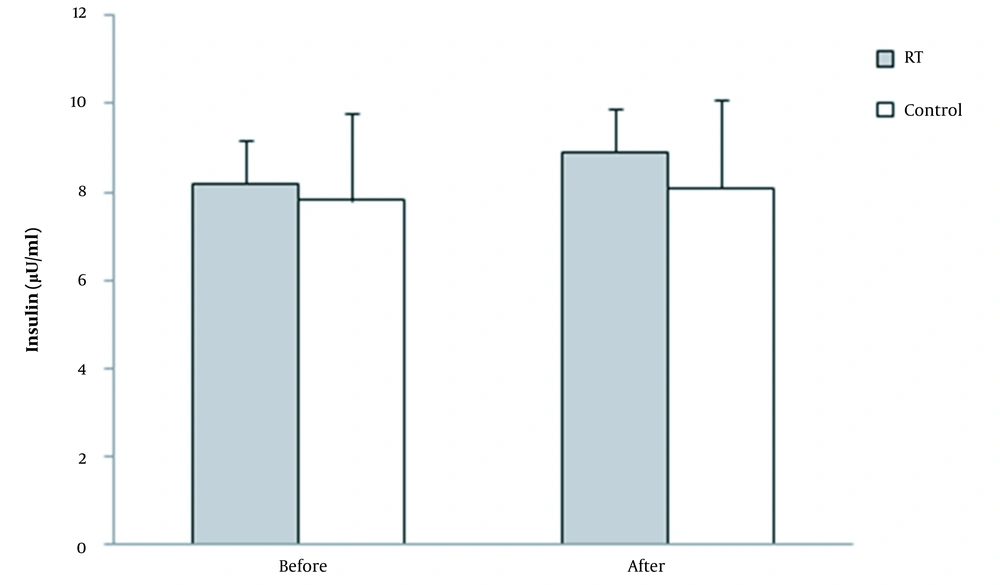
No significant changes were observed in the fasting insulin levels of both groups (RTG: from 8.2 to 8.9 µU/mL, CG: 7.8 to 8.1 µU/mL) after the training period (F = 1.1 and P = 0.6, ES: 0.16). Figure 5 presents fasting insulin levels at pre-and post-test.
4.5. Insulin Resistance
HOMA-IR levels significantly decreased (from 3.3 to 2.4) in the RTG (F = 3.5 and P = 0.05, ES: 0.38), while HOMA-IR levels approximately remained unchanged in the CG (from 3.4 to 3.5) after the training period. Figure 6 presents HOMA-IR levels at pre-and post-test.
5. Discussion
The progression of DKD is influenced by several biological factors, of which IGF-I is significantly involved in the development of DN, which regulatory effects highly depend on its binding proteins that contribute to the pathology of a diabetic kidney disorder (6, 17). The most abundant IGFBP species in the human peripheral circulation is IGFBP-3 (%80), which promotes apoptosis and insulin resistance. Hence, considering the importance of this biomarker in the prediction and development of DN, this study raises this question, “to what extent the intervention of an 8-week RT would alter the plasma levels of IGFBP-3 in DN patients”. With respect to the demonstrated effectiveness of exercise intervention in the improvement of DN and the results of previous studies indicating that the levels of IGFBP-3 are significantly higher in diabetic patients with kidney impairment compared to diabetic patients without kidney dysfunction (7, 17), the plasma levels of IGFBP-3 were expected to decrease in response to eight weeks of RT. However, contrary to our hypothesis, we observed the absence of any significant changes in the plasma levels of IGFBP-3 in the RTG. In agreement with our findings, Nindl et al. reported that there were no significant changes in free IGFBP-3 levels after 12 weeks of RT in end-stage renal disease patients (29). The same outcomes have also been reported by Borst et al. over the first phase (13 weeks) of RT in both the 1-SET and 3-SET groups, while in the second phase of RT (13 - 25 weeks), the plasma levels of IGFBP-3 significantly reduced by 20% in the 3-SET group (30). Although the exact mechanisms, by which RT leads to IGFBP-3 response are not fully understood, according to the available data, IGFBP-3 response may depend on the duration and volume of exercise. Therefore, the duration and volume of RT in this study were probably inadequate to affect the IGF-I system and alter the plasma levels of IGFBP-3.
However, with respect to the significant increase in the levels of IGFBP-3 and also the significant decrease in the eGFR levels observed in the CG, it can be proposed that IGFBP-3 response is associated with kidney function, and therefore, the lack of any change in the rate of eGFR is probably responsible for the lack of any significant changes in the levels of IGFBP-3 in the RTG. In line with this, previous studies have shown that the levels of IGFBP-3 are negatively associated with the eGFR levels in patients with DN (7). This means that the development of kidney dysfunction (a decrease in the eGFR levels) may be accompanied by an increase in the levels of IGFBP-3. Accordingly, by a simple analogy between the results of the CG and RTG, it can be logically concluded that RT, though it did not improve kidney function, at least stopped the development of kidney dysfunction and prevented eGFR from decreasing in the RTG, whereas the kidney function of the CG probably deteriorated due to the lack of RT, resulting in the increased levels of IGFBP-3. This justification can be supported by the report of Santos et al. who showed that RT could attenuate renal dysfunction in animal DN after an 8-week training period (31). However, it seems that the improvement of kidney function relies on various sophisticated physiological adaptations, requiring the long-term intervention of proper exercise therapy; otherwise, significant improvement can be barely observed through short-term training programs. In agreement with the importance of training duration in the improvement of kidney function, Ishikawa et al. reported no significant differences in eGFR levels between the exercise and control groups after exercise intervention (eight weeks) on diabetic rats with nephropathy (32). Another study also found no significant change in eGFR levels after 12 weeks of resistance and aerobic exercises (33), while Greenwood et al. observed significant improvement in the eGFR levels of 20 patients with CKD stages 3 - 4 after 12 months of high-intensity resistance and aerobic training (34). On the other hand, Hiraki et al. reported no significant differences between the eGFR levels of the control or training group after a one-year of resistance and aerobic training (35). It is worthy of note that the intensity of training protocol in the study by Hiraki et al. was moderate, while in that of Greenwood et al., the subjects underwent a one-year combined resistance and aerobic training with 80% 1RM and maximum heart rate (HR) (34). Accordingly, in addition to the duration, the intensity of RT seems to be a very important factor that affects the improvement in eGFR levels; however, Hiraki et al. (35) had a different opinion. Because muscle strength and mass are increased in response to RT, and increased muscle mass affects creatinine plasma levels, they argue that the eGFR levels measured from the plasma level of creatinine after RT may not precisely show kidney function improvement induced by exercise. It is an undeniable fact that RT increases muscle mass and strength (35). Perhaps, utilizing cystatin C or other factors that are not influenced by RT adaptations (increased muscle mass) could be more useful for future research.
Fasting blood glucose decreased after eight weeks of RT in this study. Egger et al. reported similar findings in T2D patients after eight weeks of various types of RT (36). Since RT highly relies on the energy produced by the glycolytic pathway, it was not surprising that the present study observed reductions in blood glucose levels. However, it has been suggested that people with T2D usually have a defective insulin-dependent pathway, which is responsible for activating glucose transporters of the muscles to help move the glucose from the blood into the cells (37). In this study, no significant changes were observed in fasting insulin, and therefore, the decreased levels of fasting glucose were probably the result of non-insulin-independent glucose uptake. This possibility agrees with the results of previous studies reporting an increase in glucose transport after regular muscle contraction via a contraction-stimulated pathway (37). In addition, since insulin action is closely linked to lean body mass as the primary metabolic target tissue for glucose metabolism, the increased lean body mass due to RT intervention may be another reason for decreased levels of fasting glucose in this study (38). An increase in muscle mass as the result of RT leads to increased glucose storage (39). Accordingly, it is reasonable to speculate that the small increase in muscle mass as the result of RT may be responsible for the improved glucose homeostasis and metabolism observed in the RTG (21). Nevertheless, increased capillary density, increased number of glucose-carrying proteins (GLUT4), increased glycolytic and oxidative enzyme activity, and increased glycogen activity synthesis are other related mechanisms that may affect the delivery of glucose from the blood to the muscle, decreasing the fasting glucose (40).
RT also induced a significant decrease in insulin resistance. Although the exact mechanisms for HOMA-IR reduction observed after RT in this study are not fully understood, the association between physical inactivity and insulin resistance has been suggested over the last five decades. It has been previously stated that a reduction in visceral and abdominal fat is a key linkage between exercise and insulin resistance improvement (41). In this study, although abdominal fat mass was not directly assessed, previous studies have supported the reduction of visceral and subcutaneous fat after RT (21). Hence, it may be partly justified why we observed HOMA-IR reduction only in the RTG. However, the lack of strict diet control and blood pressure monitoring were the limitations of the present study.
5.1. Conclusions
In conclusion, eight weeks of RT did not improve the renal function of T2D patients with the risk of nephropathy and had no significant effect on plasma levels of eGFR and IGFBP-3. Nevertheless, its positive role in preventing the development of renal dysfunction into DN is undeniable because the kidney function of the CG deteriorated over the research period. In addition, after eight weeks of RT intervention, blood glycemia significantly decreased in the RTG. Due to the importance of glycemic control for the treatment of T2D and its related complications, in particular DN, RT seems to be a very useful, cost-effective, and safe therapeutic measure to assist patients suffering from this disease. Although the subjects of this study were middle-aged men, it seems that RT is beneficial not only for these people but also for people of different ages and genders. However, conducting further studies on a larger sample, both genders, with a longer training period and blood pressure monitoring can better clarify the probable effects of RT on renal function of patients with T2D.
![Plasma levels of IGFBP-3 before and after eight weeks of resistance training (RT) [* Significant differences within group (P < 0.05)]. Plasma levels of IGFBP-3 before and after eight weeks of resistance training (RT) [* Significant differences within group (P < 0.05)].](https://services.brieflands.com/cdn/serve/3170b/08108194e667c34b87395142a3859d05e767d568/mcj-128713-i002-F2-preview.webp)
![eGFR value before and after eight weeks of resistance training (RT) [* Significant differences within group (P < 0.05)]. eGFR value before and after eight weeks of resistance training (RT) [* Significant differences within group (P < 0.05)].](https://services.brieflands.com/cdn/serve/3170b/088ff3ff979eaaefea656b3935ea965b1689d40a/mcj-128713-i003-F3-preview.webp)
![Fasting blood glucose levels before and after eight weeks of resistance training (RT) [* Significant differences within group (P < 0.05); † Significant differences between groups (P < 0.05]. Fasting blood glucose levels before and after eight weeks of resistance training (RT) [* Significant differences within group (P < 0.05); † Significant differences between groups (P < 0.05].](https://services.brieflands.com/cdn/serve/3170b/5262a909e6cf3b2ebea82ba18347ce2336c1d65b/mcj-128713-i004-F4-preview.webp)
![Insulin resistance index (HOMA-IR) levels before and after eight weeks of resistance training (RT) [* Significant differences within group (P < 0.05); † Significant differences between groups (P < 0.05)]. Insulin resistance index (HOMA-IR) levels before and after eight weeks of resistance training (RT) [* Significant differences within group (P < 0.05); † Significant differences between groups (P < 0.05)].](https://services.brieflands.com/cdn/serve/3170b/eaeb293f5a34ed67c126bf22978b0fc1135976a1/mcj-128713-i006-F6-preview.webp)