1. Background
Glioblastoma (GBM) is an exceedingly aggressive brain cancer that develops from neural stem cells, astrocytes, and oligodendrocytes with a 6.7% 5-year survival rate (1). Gliomas are classified into four grades based on the extent of anaplasia: grade I, non-infiltrating astrocytomas; grade II, diffuse astrocytomas; grade III, anaplastic astrocytomas; and grade IV, most aggressive form of GBM (2). According to the WHO's categorization of brain tumors, gliomas are classified as grade IV and have an incidence of 3.19 instances per 100,000 people (3). Epidemiological evidence has indicated that the incidence rate of primary brain tumors (PBTs) is the highest among those younger than 14 and older than 70 (4). However, the rationale for increasing PBTs among mentioned age groups remains unclear. Numerous research has been undertaken in recent years to identify risk factors for brain tumors. Brain tumors can be generated by positive family history, exposure to infectious agents such as viruses, brain trauma, smoking and alcohol consumption, toxic substances, and exposure to ionizing radiation (5). Moreover, the most important underlying causes of brain tumors are the alternating expression of genes involved in inflammation, oxidative stress, cell death, proliferation, survival, invasion/metastasis, cancer stemness, drug resistance, repair, and stability of DNA structure (4). Based on the literature review, overexpression of oncogenes like RAS (rat sarcoma), PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha), CDK4 (cyclin-dependent kinase 4), MDM2 (murine double minute 2), and EGFR (epidermal growth factor receptor), or loss of function of tumor suppressor genes like P53 (tumor protein P53), PTEN (phosphatase and tensin homolog), CDKN2A (cyclin-dependent kinase inhibitor 2A), and RB (retinoblastoma), may occur as a result of genetic and epigenetic alterations that trigger glioma tumor formation. Diffuse malignant astrocytoma might form with the loss of P53 function and the subsequent activation of the RAS pathway due to NF1 inactivation (6). On the other hand, evidence has shown EGFR overexpression, loss of heterozygosity on chromosome 10q, mutations in p53 and PTEN, and absence of IDH1 (isocitrate dehydrogenase-1) are hallmarks of primary GBM (7). All CNS (central nervous system) cell types depend on EGFR signaling for their maintenance, proliferation, migration, and differentiation. Overexpression of the receptor/ligand, receptor mutation, and other processes contribute to EGFR dysregulation in GBM (8). Anti-inflammatory cytokines, most notably TGF-β (transforming growth factor-β) and IL-10 (interleukin-10), are overexpressed in GBM in several investigations (9). In the setting of cancer, IL-10 is a crucial pleiotropic immunoregulatory cytokine that exerts immunosuppression by downregulating antigen-presenting cells (APCs), preventing T cell proliferation, and activating the activity of regulatory T (Treg) cells (10). However, the therapeutic potential of targeting this cytokine in the setting of brain cancer and involvement of IL-10 in the tumor microenvironment remains elusive. Huettner et al. published the first evidence for IL-10 expression in glioma, showing that high-grade (grade III and IV) gliomas express higher quantities of IL-10 mRNA than lower-grade (grade II) gliomas (11). On the other hand, cell death arrest as a critical mechanism in the cancerous process may be triggered, thereby making cancer cells aggressive. Moreover, death receptor-induced apoptosis was first linked to Casp8 (caspase 8) (12). Casp8 expression is downregulated, and apoptosis signaling is missing in most cancers (13). However, glioma patients with elevated caspase-8 expression may have a worse prognosis. Casp8 may enhance the production of nuclear factor kappa B (NF-κB)-dependent proangiogenic cytokines and tumor promoters in glioma models (14). Feng et al. discovered that irradiated glioma cells in their latter stages of life triggered a Casp3-dependent NF-κB/cyclooxygenase-2 (COX-2)/prostaglandin E (PGE) axis to create a proangiogenic milieu (15). These findings indicated that glioma, like other malignancies, might revert the NF-κB-dependent pro-apoptotic action of Casp8 or Casp3, encouraging blood vessel creation (16).
Today, there are a variety of therapies for PBT (primary brain tumors), including chemotherapy, surgery, targeted drugs, and radiation therapy, some of which have life-threatening side effects (17). Tumor excision followed by radiation and chemotherapy with temozolomide, and more recently, tumor-treating (TT) fields, are all examples of currently approved treatments. Several resistance mechanisms, such as insufficient chemotherapeutic drug penetration of the blood-brain barrier and an increased likelihood of temozolomide resistance in a subset of patients, pose significant challenges to existing treatment approaches (1). Therefore, there is a need to find lower-risk approaches and complementary and conventional therapies to reduce the adverse effects. Complementary and alternative medicines (CAMs) are medications and health practices not generally utilized by the medical field to treat cancer (18). However, complementary medicine combined with commercial/conventional pharmaceuticals can be the most achieved as combinational medical therapy in pathogenic hallmark offset (19). The conventional medical treatment combines CAMs techniques established to be safe and effective by scientific research (20).
Exercise training has been identified as an affordable, simple, and effective therapy for preventing and treating a variety of inflammatory responses, oxidative stress, cell death, proliferation, survival, and invasion/metastasis (21). Despite the complications of conventional treating brain tumors, immense studies have revealed that aerobic training may favorably and psychologically benefit patients by decreasing tumor size, invasion/metastasis, drug resistance, and inflammation and raising their survival rate (20, 22, 23). Physical exercise has been recognized to minimize the occurrence and recurrence of malignancies and increase survival through increasing immunocompetent. In most of the evidence, it has been observed that swimming exercises may decrease the formation of malignant cells (24, 25).
Moreover, ample evidence indicated that swimming aerobic exercise could improve neurogenesis and cognition, promote nerve anti-inflammatory and regrowth in the peripheral nervous system, reduce neuropathic pain, and improve muscle mass and function (in mice models research) (23, 26). As a result, exercise treatments offer great promise as adjunctive therapy for nerve damage among mice.
Curcumin, a bioactive component of turmeric, has been studied for its potential as an anti-inflammatory and antitumor herbal medication. Curcumin is an antioxidant that has shown promise in reducing tumor growth and blocking metastasis (27). Also, there is growing evidence that green tea, which has an antioxidative and anticancer impact, may help prevent the development of certain malignancies (28). In addition, ginseng's chemoprotective action allows for various medicinal applications. Medicinal effects on the anti-toxicity and immune system have been suggested by preliminary studies of ginsenosides, an antioxidant substance found in ginseng (29). Besides, the herb Matthiola incana has been indicated to be effective as a tumorigenesis inhibitor. It should be noted that the molecular mechanism by which this plant exerts its anticancer effects, including its antioxidative stress, cytotoxic effects, and antioxidants, is not yet completely understood (30).
2. Objectives
This study mainly aimed to appraise the effects of swimming training and a mix of herbal drugs (extract and nanoliposome forms) as a complementary strategy to improve sensory-motor impairment features in rats with brain tumors.
3. Methods
3.1. In Silico Analysis
Significant genes and molecular signal transduction pathways associated with brain malignant tumour pathogenesis and clinical side hallmarks, such as impaired sensory-motor integration, were identified using molecular biological analysis. We pulled the closest data set with ID number GSE78895 for the brain tumor in the gene expression omnibus (GEO) database. We gathered mid-brain-related data for bioinformatics evaluation among various brain areas. We used the R programming language, its associated limma and s4vector packages, and MAS5 normalization. We used a heat map plot to highlight significant genes having a P-value less than 0.001. Moreover, we browsed the DisGeNET database to obtain the PBTs gene-disease association list with reference No. C0750974.
3.2. Ethical Code
This study was conducted based on the ARRIVE guidelines and was performed and approved according to protocols for laboratory animals by the Ethics Committee of Islamic Azad University, Khorasgan Branch (IR.IAU.KHUISF.REC.1400.335).
3.3. Animal Working and Treatment
In this experimental study, 56-male-Wistar rats were purchased (230 ± 20g) from Pasteur Institute (Karaj, Iran). The rats were kept under conventional conditions (60 ± 5 percent relative humidity, 23 ± 4°C, and 12 h dark/12 h light cycle). The animals had free access to food and water. Moreover, the rats were divided into eight groups (n = 7): (1) Normal group: The rats did not receive supplements and apply exercise training; (2) model group: Injections of the C6 glioma cell line and stereotaxic techniques were used to induce a brain tumor model in the rats; (3) model + exe group: The rats were induced brain tumor model (BT model) and were subjected to swimming training; (4) model + lipo: BT model received nanoliposomes (without extract); (5) model + extract: BT model received a complex complement with four plant extracts (Ginseng, M. incana, Turmeric, and Green-Tea); (6) model + lipo-extract: BT model received nanoliposomes enriched complex complement with four plant extracts; (7) model + extract-exe: BT model received complex complement with four plant extracts and applied swimming training; (8) model + lipo-extract + exe: BT model received nanoliposomes enriched complex complement with four plant extracts and swimming training.
3.4. Brain Tumor Modeling
Male-Wistar rats were injected with the C6 glioma cell line (5 × 105 in 10 μL cell suspension) in the substantia nigra (SN) area (deep ventral (DV): -7.6 mm, anterior-posterior (AP): -5.5 mm, medial-lateral (ML): -2.4 mm; anterior-posterior (AP): -5.2 mm, medial-lateral (ML): -1.9 mm; deep ventral (DV): -8.2 mm (31). The C6 glioma cell line was injected under ketamine/xylazine (3: 5) anesthesia. Following surgery, the animals were extensively observed (body temperature, wound healing, and behavior). After vaccination, the animals were kept in separate cages. It should be noted that, in this study, we did not lose study samples.
3.5. Consumption of Nanoliposomes and Herbal Extraction
The final dosage of compounds (100 mg/kg, once per day) in one ml of normal saline was dissolved and gavaged five days a week for six weeks (32).
3.6. Protocol of the Swimming Training
Swimming training was performed on rats for a duration of time six weeks. Rats swam three times weekly for ten minutes to acclimate to the environment and the pool. The rats were then subjected to 3-day/week, thirty-minute, during a six-week swimming training regimen. In addition, the temperature of water was 22 - 25°C (33). Based on the literature analysis and World Health Organization (WHO) recommendations, the training protocol's intensity was moderated (34).
3.7. Nanoliposomes Delivery System Preparation Guideline for Polyherbal Extract
Research into these conditions has focused on discovering what sets them apart biologically and discovering new ways to treat them clinically, such as by reversing the dopamine deficit (35). The herbal medicine center acquired 100 grams of ginseng, green tea, turmeric, and M. incana to prepare the polyherbal extract. First, the plants were cleaned with distilled water and dried at 40 degrees Celsius for seven days. Electric mills were used to pulverize dry herbs for the mixing extraction, and a hydroalcoholic solution (70: 30) was supplied at 40°C for 48 hours. The solution was then liquefied and filtered using a Buchner funnel. It was then placed in a distillation system with a rotary rotator at 50 RPM and 50°C for 45 to 50 minutes. To achieve a final concentration of 1 mM, the herbal extract was diluted in sterile distilled water. After dissolving one gram of phosphatidyl colin in ethanol, a rotavapor was used to generate a thin lipid layer on the flask's wall. The organic solvent was then evaporated under vacuum completely, and the suspension was stirred for two hours under nitrogen. The samples were then sonicated for 360 seconds at 40 kHz and 40% maximum power to get a homogeneous solution (1 s on, 1 s off) (36). Liposome samples were stored in opaque glass bottles at 4°C. In addition, the average size, electrophoretic mobility, and zeta-potential of liposomes were determined using DLS and empirical dilutions with a Malvern Zetasizer Nano ZS (Malvern Instruments Ltd., UK, ZEN3600) and DTS Nano software version 6.12. The data were presented as vesicle size, polydispersity index, and zeta potential. Liposomes were synthesized in triplicate (n = 3) following three runs (37). Scanning electron microscopy was used to determine the morphological characteristics of nanoliposome particles (SEM, LEO 1430VP, Germany and UK). The nanoparticles were provided by Histogenotech Company, Tehran, Iran.
3.8. Assessment of the Gene Expression Level
The TRIzol reagent was used following the manufacturer's instructions to isolate total RNA (Thermo Scientific, USA). RNA sample purity and concentration were confirmed in OD 260/280 nm by NanoDrop analysis (Thermo Scientific, Waltham, MA, USA). In addition, cDNA synthesis was undertaken according to the instructions (TaKaRa, Kusatsu, Shiga Prefecture, Japan). In addition, SYBR Green dye and quantitative real-time PCR were utilized to assess gene expression (Rotor-Gene 6000 instrument, Corbett Life Science, Mortlake, Australia). We used GAPDH as a normalization reference gene. A statistical study determined fold changes in mRNA expression using the 2−ΔΔCt method. The list of primers is shown in Table 1.
| Gene | Sequence of Primers | Size (bp) |
|---|---|---|
| P53 | Forward: 5’- CCGACTATACCACTATCCACTAC -3’ | 147 |
| Reverse: 5’- CACAAACACGAACCTCAAAGC -3’ | ||
| Hras | Forward: 5’- GTTTGGCAGCCCCTGTAGAA -3’ | 132 |
| Reverse: 5’- CTATAGTGGGATCATACTCG -3’ | ||
| IL-10 | Forward: 5’- GCAGGACTTTAAGGGTTACTTGG -3’ | 181 |
| Reverse: 5’- GGGGAGAAATCGATGACAGC -3’ | ||
| Casp8 | Forward: 5’- GTAAACTTTGGCGGACTG -3’ | 303 |
| Reverse: 5’- AGCCTCTGAAATAGCACC -3’ | ||
| GAPDH | Forward: 5’- AGGTCGGTGTGAACGGATTTG -3’ | 123 |
| Reverse: 5’- TGTAGACCATGTAGTTGAGGTCA -3’ |
The Sequence of Oligonucleotide Primers
3.9. Behavioral Tests
3.9.1. Beam Test
The Beam Test is used to measure to assess motor coordination. In the first stage, the animals are placed in the corner of the bar and allowed to walk from one end to the other through the narrow bar at least three times. The narrow rod is 1 - 3 cm wide and is placed on a platform and rat cage. This test records the number of foot slips and the time required to cross the bar. It should be noted that if the animal did not move on the rod, it could be stimulated to walk by tapping the tail. Notably, the healthy rats could pass the bar without additional support, but rats with impaired forelimb or hind limb function were often placed on the restraint as support.
3.9.2. Sciatic Functional Index
The Sciatic Functional Index (SFI) is a practical method for measuring the recovery of sciatic nerve function in rats after various experimental lesions and therapies. Although this technique is objective, it relies on the examiner's ability to correctly identify and note the predetermined landmarks of the footprint (38). This channel is made of wood and measures 50 cm high, 7 cm wide, and 60 cm long. A length of 60 cm is chosen to obtain at least 5 - 7 footprinting, a width of 7 cm to prevent the animal from going around while moving, and a height of 50 cm to construct relative darkness inside the channel. During the midstance phase, measurements were obtained from footprint photos by manually identifying each toe. The length from the heel to the third toe (print length), the distance from the first toe to the fifth toe (toe spread), and the distance from the second toe to the fourth toe were measured for each footprint (intermediate toe spread). In the experimental and control rat models, all three measurements were taken. Using the methodology proposed by Varejão et al., the following sciatic functional index was determined (38):
PL represented the print length, TS represented the toe spread, ITS represented the intermediate toe spread, E represented the experimental model, and N represented the normal model. A score of 100 demonstrated total disability, whereas a score of zero indicated normal function.
3.10. Statistical Analyses
The sample size was estimated based on 80% power and an alpha level of 0.05. Statistical analysis was conducted using GraphPad Prism (version 9; GraphPad software). The Shapiro-Wilk test was used for normalizing distribution, and variables were normally distributed. Moreover, we detected the effects of the intervention and the interaction effects of the interventions based on the two-way analysis of variance (ANOVA) with Tukey's post hoc test due to multiple comparisons. In addition, a P-value < 0.05 difference was judged significant. Also, the data are displayed as mean and standard deviation (SD). The statistical analyses of weight changes were calculated by one-way ANOVA with repeated measures followed by Tukey's post hoc.
4. Results
4.1. Bioinformatics Assessment
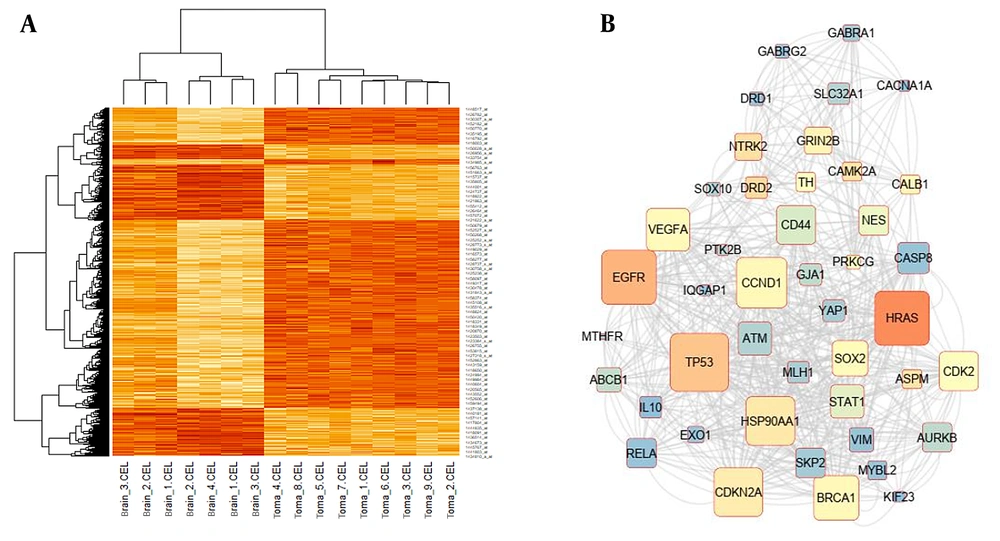
The microarray dataset of midbrain cancer identified 20,784 genes with significantly differential expression compared to normal midbrain samples (P-value < 0.05). Differential gene expression with a P-value less than 0.001 was depicted in the heatmap plot by R statistical data analysis software (Figure 1A). The differential expression profiles of influential genes in brain cancer revealed that 583 were overexpressed and 569 downregulated at the log FC ± 4 cut-off compared to normal midbrain samples. Network analysis using the metrics degree = 20, betweenness centrality = 0.002, and closeness centrality = 0.2 identified 44 hub genes with causal effects involved in brain tumor etiology. The genetic interactions network of hub genes designed by CytoScape 3.6.0 software was displayed in Figure 1B.
A, The heat Map visualization tool in the R statistical language analysis displayed remarkably differential gene expression patterns in patients with midbrain cancer compared to healthy controls using the (P < 0.001 thresholds); B, Hub genes considered to have a role in the progression of midbrain cancer were mapped out in a protein-protein interactions network and sorted according to the network's features such as degree, betweenness centrality, and closeness centrality.
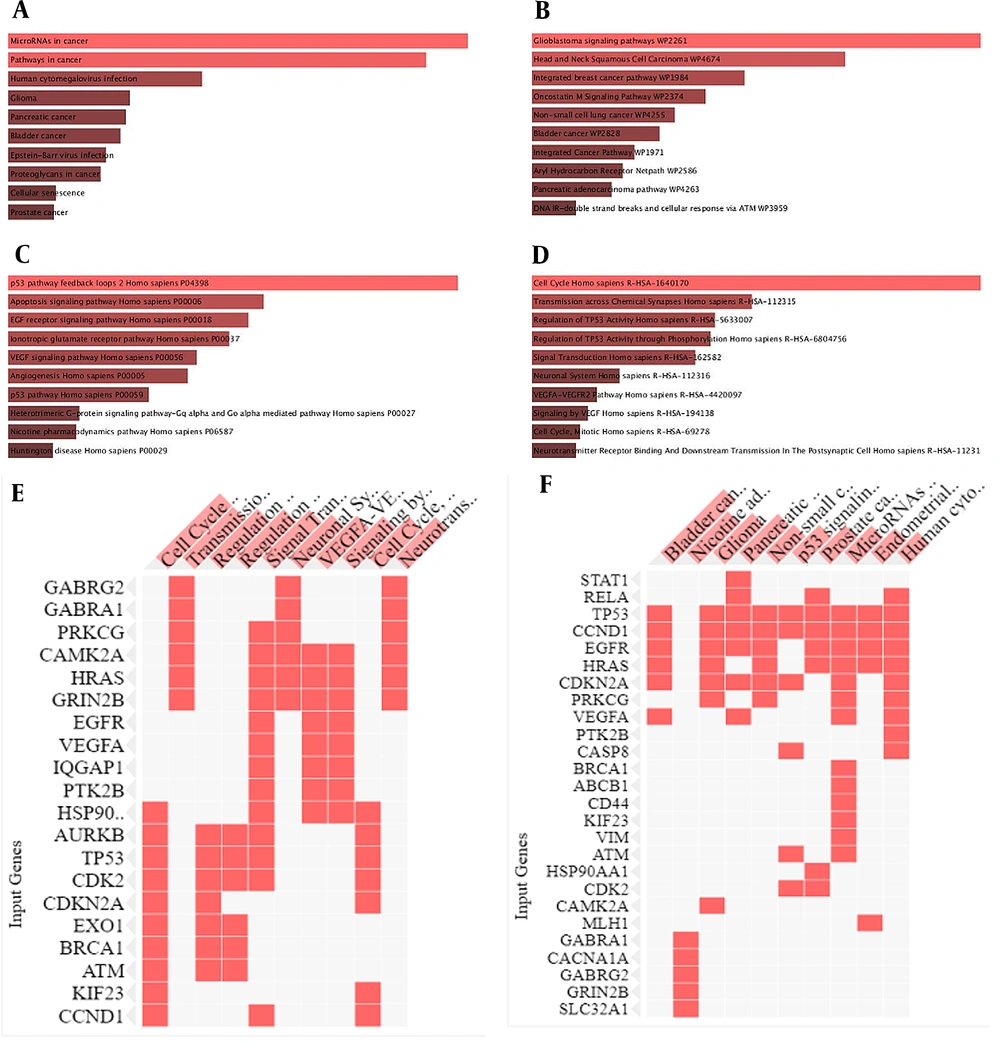
Cellular senescence, the P53 pathway feedback loop, apoptosis, EGF receptor signaling, vascular endothelial growth factor (VEGF) signaling, angiogenesis, transmission across a chemical synapse, neurotransmitter receptor binding, and downstream transmission in the postsynaptic cell, GBM, glioma, and neurodegenerative diseases signaling patterns were highlighted by the Enrich-r algorithm (Figure 2A - F). Hence, we suggested P53, Hras, IL-10, and Casp8 as hub genes in mid-brain tumor pathogenesis with movement instability signs appointed by bioinformatics analysis.
Major molecular signaling pathways are attributed to hub genes. A – F, Hub genes were analyzed using the Enrich r intelligent server. The genes played a role in a variety of processes, including cellular senescence, the P53 pathway feedback loop, apoptosis, EGF receptor signaling, vascular endothelial growth factor (VEGF) signaling, angiogenesis, transmission across a chemical synapse, neurotransmitter receptor binding, and downstream transmission in the postsynaptic cell. Major molecular signaling pathways collaborate with hub genes in the pathogenesis of GBM.
4.2. Qualities of Nanoliposomes
After sonicating the nanoliposome, we assessed the particle dimensions on days 0 and 30. The nanoliposome particle size at either 4°C or 37°C showed significant stability. Furthermore, the generated nanoparticles had an electric charge of -3.39 microvolts, as measured by the potential zeta test. So, we concluded that the nanocoatings were stable because they were negatively charged. According to DLS findings, nanoparticles range in size from 60 nm to 600 nm, with the majority having a size of 100 nm. Because of their spherical shape and high density, the SEM microscopic examination findings corroborate the size of the nanoparticles created, as evidenced by the scale bar in the DLS test (Table 2).
| Zeta Potential (mV) | Mobility (μmcm/Vs) | DLS Test (nm) | Nanoliposome Average Size (nm) |
|---|---|---|---|
| -3 - 39 | -0.2655 | 60 - 600 | 100 |
Nanoliposomes’ Characteristics
4.3. Swimming Training and Nanoliposomes Enriched Combined Supplement Regulated the Inflammasome Activation
We found that the relative expression of the IL-10 and Casp8 significantly increased in the model group compared with the normal group (Figure 3A and B). Moreover, swimming training (model + exe group) and complex complement with four plant extracts (ginseng, M. incana, turmeric, and green-tea) (model + extract) regulated the expression of IL-10 and Casp8 compared with the model group (Figure 3A and B). Notably, this study indicated that nanoliposomes enriched complex complement significantly decreased the expression level of IL-10 and Casp8 compared with Model and complex complement with four plant extracts groups (Figure 3A and B). Based on these data, we demonstrated that nanoliposomes enriched complex complement had a more practical function than plant extracts (Figure 3A and B). Furthermore, the brain tumor rat models, which received a complex complement with four plant extracts and applied swimming training (model + extract-exe), reduced the expression level of IL-10 and Casp8 genes compared with the model group (Figure 3A and B). Besides, the BT model received nanoliposomes enriched complex complement, and swimming training (model + lipo-extract + exe) significantly modified these genes compared with the other groups. Hence, we explored that the swimming training and nanoliposomes enriched combined supplement ameliorated the inflammasome activation in the brain tumor rat models.
Swimming training and nanoliposomes enriched combined supplement improved gene expressions. a-d: the relative expression of IL-10, Casp8, P53, and Hras [data expressed as mean ± SD. + indicates statistically significant difference with normal group, * indicates statistically significant difference with Model group, @ indicates statistically significant difference with Model + Liposome group, # indicates statistically significant difference with Model + EXE group, & indicates statistically significant difference with model + extract group, ! indicates statistically significant difference with model + liposomal extract group, ^ indicates statistically significant difference with model + exe + extract group, $ indicates statistically significant difference with model + exe + liposomal extract. The data were calculated by one-way analysis of variance (ANOVA) with Tukey's post hoc test. The effects of the intervention and the interaction effects of the interventions were detected based on the two-way analysis of variance (ANOVA) with Tukey's post hoc test due to multiple comparisons].
4.4. Swimming Training Along with Nanoliposomes Enriched Combined Supplement Modified P53 and Hras Expression
The expression level of P53 and Hras decreased in the brain tumor rat models compared with the normal group (Figure 3C and D, Table 3). Also, the brain tumor rat models subjected to swimming training (model + exe group) and the brain tumor rat models who received a complex complement with four plant extracts (model + extract) significantly enhanced the relative expression of P53 and Hras compared with the Model group (Figure 3C and D). In addition, our data showed that the expression level of P53 and Hras was significantly upregulated in the model + lipo-extract group compared with model and complex complement with four plants extracts groups (Figure 3C and D, Table 3). Interestingly, the model + lipo-extract + exe group significantly amplified P53 and Hras genes compared with the other groups (Figure 3C and D, Table 3). Based on these data, we could conclude that nanoliposomes enriched complex complement and swimming training modified and regulated the tumor suppressor and cell division in the brain tumor rat models.
| Groups | IL-10 | P-Value | Casp8 | P-Value | P53 | P-Value | Hras | P-Value |
|---|---|---|---|---|---|---|---|---|
| Normal | 0.98 ± 0.038 | 1.015 ± 0.13 | 1.054 ± 0.09 | 1.05 ± 0.09 | ||||
| Model | 7.66 ± 0.68 b | < 0.0001 | 9.06 ± 0.71 b | < 0.0001 | 0.112 ± 0.02 b | < 0.0001 | 0.04 ± 0.03 b | < 0.0001 |
| Model + lipo | 7.33 ± 0.31 c | 0.9987 | 9 ± 0.6 c | > 0.9999 | 0.114 ± 0.019 c | > 0.9999 | 0.043 ± 0.02 | > 0.9999 |
| Model + exe | 6.33 ± 0.15 c | 0.0253 | 7.333 ± 0.15 c | 0.0009 | 0.31 ± 0.03 c | < 0.0001 | 0.35 ± 0.039 c | < 0.0001 |
| Model + extract | 5.2 ± 0.26 c | < 0.0001 | 6.2 ± 0.26 c | < 0.0001 | 0.3933 ± 0.03 c | < 0.0001 | 0.43 ± 0.042 c | < 0.0001 |
| Model + lipo-extract | 3.33 ± 0.42 c | < 0.0001 | 4.6 ± 0.3 c | < 0.0001 | 0.5367 ± 0.05 c | < 0.0001 | 0.55 ± 0.03 c | < 0.0001 |
| Model + extract-exe | 2.07 ± 0.21 c | < 0.0001 | 3.333 ± 0.42 c | < 0.0001 | 0.68 ± 0.03 c | < 0.0001 | 0.75 ± 0.045 c | < 0.0001 |
| Model + lipo-extract + exe | 1.53 ± 0.32 c | < 0.0001 | 2.5 ± 0.4 c | p < 0.0001 | 0.8433 ± 0.04 c | < 0.0001 | 0.88 ± 0.04 c | < 0.0001 |
The Relative Expression in Each Group a
4.5. Nerve Function was Improved by Swimming Training Along with Nanoliposomes Enriched Combined Supplement
Based on the SFI and beam test, the nerve function and motor-sensory of the brain tumor rat models were reduced compared with the normal group (Figure 4A and B, Table 4). Moreover, the SFI and beam scores were improved in the model + exe and model + extract groups (Figure 4A and B, Table 4). In addition, we found that the scores of the SFI and beam tests significantly ameliorated in the brain tumor rat model received nanoliposomes enriched complex complement (model + lipo-extract group) and rat model received nanoliposomes enriched complex complement and swimming training (model + lipo-extract + exe group) (Figure 4A and B, Table 4). Based on these behavior tests, the results indicated that the nerve function was improved by swimming training along with nanoliposomes enriched combined supplement. Moreover, we indicated that the weight of the Model group significantly decreased compared with normal group. Also, we demonstrated that model + lipo-extract + exe group improved the weight of the rats (Table 5).
The effect of swimming training and nanoliposomes enriched combined supplement on the behavior tests [data are expressed as mean ± SD. + indicates statistically significant difference with Normal group, * indicates statistically significant difference with model group, @ indicates statistically significant difference with model + liposome group, # indicates statistically significant difference with model + exe group, & indicates statistically significant difference with model + extract group, ! indicates statistically significant difference with model + liposomal extract group, ^ indicates statistically significant difference with model + exe + extract group, $ indicates statistically significant difference with model + exe + liposomal Extract. The data were calculated by one-way analysis of variance (ANOVA) with Tukey's post hoc test. The effects of the intervention and the interaction effects of the interventions were detected based on the two-way analysis of variance (ANOVA) with Tukey's post hoc test due to multiple comparisons].
| Groups | Sciatic Functional Index | P-Value | Beam Test | P-Value |
|---|---|---|---|---|
| Normal | -22.0 ± 2.646 | 20.67 ± 2.082 | ||
| Model | -75.33 ± 5.132 b | < 0.0001 | 2.9 ± 0.3606 b | < 0.0001 |
| Model + liposome | -74.33 ± 4.04 c | > 0.9999 | 3.233 ± 0.2309 | 0.9996 |
| Model + exe | -60.0 ± 2.0 c | 0.0002 | 6.250 ± 0.3536 c | 0.0100 |
| Model + extract | -54.33 ± 2.3 c | < 0.0001 | 7.333 ± 0.2887 c | 0.0002 |
| Model + liposomal extract | -46.33 ± 3.055 c | < 0.0001 | 10.50 ± 0.5 c | < 0.0001 |
| Model + exe + extract | -37.0 ± 1.0 c | < 0.0001 | 13.07 ± 0.4041 c | < 0.0001 |
| Model + exe + liposomal extract | -27.33 ± 0.58 c | < 0.0001 | 16.0 ± 0.5 c | < 0.0001 |
The Behavior Tests a
| Groups | One Week | Two Week | Three Week | Four Week | Five Week | Six Week | P-Value |
|---|---|---|---|---|---|---|---|
| Normal | 232 ± 4.6 | 235 ± 3.4 | 235 ± 6.2 | 240 ± 6.1 | 244 ± 2.5 | 250 ± 5.2 | |
| Model | 230 ± 5.4 | 227 ± 1.5 | 224 ± 2.6 | 220 ± 3.9 | 221 ± 4.4 | 215 ± 1.1 b | < 0.0001 c |
| Model + liposome | 229 ± 3.2 | 228 ± 2.1 | 226 ± 1.4 | 223 ± 2.7 | 219 ± 3.2 | 216 ± 2.7 | 0.8990 c |
| Model + exe | 231 ± 2.6 | 232 ± 1.6 | 234 ± 2.2 | 235 ± 3.1 | 237 ± 2.9 | 239 ± 2.1 d | < 0.0001 c |
| Model + extract | 230 ± 3.6 | 232 ± 2.7 | 233 ± 3.1 | 234 ± 1.9 | 236 ± 4.1 | 238 ± 2.56 d | < 0.0001 c |
| Model + liposomal extract | 229 ± 5.1 | 231 ± 3.5 | 234 ± 4.1 | 236 ± 3.2 | 239 ± 2.3 | 240 ± 3.3 d | < 0.0001 c |
| Model + exe + extract | 228 ± 4.32 | 231 ± 3.5 | 234 ± 4.1 | 236 ± 3.2 | 237 ± 2.3 | 243 ± 3.65 d | < 0.0001 c |
| Model + exe + liposomal extract | 229 ± 4.96 | 231 ± 3.5 | 234 ± 4.1 | 238 ± 3.2 | 243 ± 2.3 | 247 ± 1.2 d | < 0.0001 c |
The Weight of the Rats a
5. Discussion
In this study, we found that swimming training along with nanoliposome-enriched combined supplements could modify the expression level of the P53, Hras, IL-10, and Casp8 in the brain tumor rat models. In addition, we indicated that swimming training improved nerve function and motor-sensory, along with nanoliposomes enriched combined supplement based on the behavior tests.
The relative expression of the P53 and Hras significantly enhanced in the model + lipo-extract group compared with model and complex complement with four plants extracts groups. In addition, the expression of the IL-10 and Casp8 was decreased by nanoliposomes enriched complex complement. Feng et al. revealed that irradiated glioma cells in their latter stages of life triggered a Casp3-dependent NF-κB/COX-2/PGE axis to create a proangiogenic milieu (15). These findings show that glioma, like other malignancies, may revert the NF-κB-dependent pro-apoptotic action of Casp8 or Casp3, encouraging blood vessel creation (21). Integrative analysis evidence indicated that Casp8 could be a potential prognostic marker for glioma (15). Glioma cell migration and invasion were halted following Casp8 silenced (16). Based on the bioinformatics analysis, we found that Casp8 showed a considerable differential expression in the tumor compared to normal brain tissue; the relative expression experiment validated these findings. According to these findings, it may be recommended Casp8 expression profile be employed as a potent biomarker for following up and monitoring glioma status. Hence based on these data, we conclude that consumption of the nanoliposomes form of complex complement with four plant extracts has effective than plant extracts. G Wang and co-workers have indicated that quercetin nanoliposome could improve the JAK2/STAT3 pathways and mitochondrial function in glioma cell death.
We demonstrated that the BT model received nanoliposomes enriched complex complement and swimming training (model + lipo-extract + exe) significantly regulated P53, Hras, IL-10, and Casp8 compared with the other groups. Despite various treatment strategies for GBM status, this pathogenic condition owns a poor prognosis and diagnosis approaches (39). Due to falls, epileptic seizures, or bleeding concerns, physical activity is seldom prescribed to GBM patients. Troschel et al. arranged a high-intensity and long-term exercise program for a GBM patient. They have reported that patients with GBM receiving comprehensive multidisciplinary treatment could participate in high-intensity physical training programs. After several treatments and tumor development, these patients could continue exercising without experiencing any adverse effects. Hence, they concluded that additional research is needed rather than discouraging activity in GBM patients (40). On the other hand, a systematic review, including 15 articles, illustrated that a better quality of life and less severe side effects from brain cancer were related to greater physical activity levels. Nevertheless, the ability and dose for physical activity in GBM cancer status remain unclear (41). Moreover, Naghibi et al. suggested that regular exercise training might treat stress- and anxiety-related conditions, including progressive neurodegenerative disease (42). In this study, we had some limitations; for example, we did not measure the protein level. Moreover, this study was lack of data about the chemical therapy and compared it with complementary medicine. Swimming is a powerful way to improve aerobic endurance since it uses muscles and ligaments whole body (43). However, for researchers that employ swimming, the intensity of training is characterized by the quantity of swimming that receives place each day: low intensity (20 - 59 min/day), moderate intensity (60 - 89 min/day), and high intensity (90 min/day). Depending on the training adaption, fixed or increasing training duration may be implemented. The same standard criteria of six weeks in the treadmill exercise protocol are used to categorize swimming training workouts as either short-term (less than six weeks) or long-term (six weeks or more) (26). On the other hand, herbal medicine is one of the most popular forms of CAM (19).
In this study, we found that the weight of model groups was significantly reduced compared with normal group. Notably, we revealed that model + lipo-extract + exe group improved the weight of the rats. Moreira et al. indicated that aerobic exercise training might improve the muscle mass in GBM conditions (43).
We suggested P53, Hras, IL-10, and Casp8 as hub genes in mid-brain tumor pathogenesis with movement instability signs appointed by the bioinformatics study. Besides, the enrichment of collaborative hub genes between the GEO data set and the DisGeNET database indicated that these hub genes collaborate in the cellular senescence, the P53 pathway feedback loop, apoptosis, EGFR signaling, VEGF signaling, angiogenesis, transmission across a chemical synapse, neurotransmitter receptor binding, and downstream transmission in the postsynaptic cell, GBM, glioma, and neurodegenerative diseases signaling pathways. Neurotransmitters and brain-specific messengers function as signaling molecules that can affect the proliferation, quiescence, and differentiation of various cell types that make up the central nervous system. Neurotransmitters, by their effect on tumor progenitors, play a crucial role in the initiation and development of malignant brain tumors (44).
Moreover, a critical pleiotropic immunoregulatory cytokine, IL-10, prevents T cell proliferation, inhibits APCs, and activates Treg cells in the context of cancer, hence promoting immunosuppression (10). The first evidence of IL-10 expression in glioma was reported by Huettner et al., and it showed that gliomas of high-grade (grades III and IV) express more IL-10 mRNA than gliomas of lower grade (grade II) (11). This finding is supported by gene expression data for gliomas obtained from the Cancer Genome Atlas and the Chinese Glioma Genome Atlas (CGGA) database, which show that IL-10 mRNA expression is higher in high-grade (grades III and IV) gliomas than in low-grade (grade II) gliomas. Furthermore, based on the bioinformatics analysis, we found that IL-10 has a significant differential expression in the glioma compared to normal brain tissue; the relative expression assay confirmed these results. According to these findings, it can be proposed IL-10 expression profile used as a robust biomarker for following up and monitoring glioma status.
On the other hand, cell death arrest as a critical mechanism in the malignant process can be triggered the development and becoming aggressive of cancer cells. Death receptor-induced apoptosis is associated with Casp8 which the Casp8 expression is downregulated, and apoptotic signaling is absent in most malignancies (13). However, clinical evidence indicated that glioma patients with increased Casp8 expression might have a poorer prognosis. Casp8 may boost the production of NF-κB-dependent proangiogenic cytokines and tumor promoters in glioma models (14). In addition, its growth-promoting properties in fibrosis, wound healing, tissue regeneration, and tumor reunion have all been proven (45).
Nanoliposomes containing ginseng, M. incana, turmeric, and green tea positively impacted movement instability indexes of rat models with mid-brain tumors. Liposome carriers rank at the top terms of the lipid-based delivery system due to their wide variety in encapsulating various compounds with varying polarities. Liposome carriers are nontoxic, biodegradable, and do not irritate the body's immunological system. The advantages of liposomes are excellent encapsulation efficiency, tailored release, easy production, and high stability. Due to their greater surface area, nanoliposomes are more stable, soluble, and bioavailable than liposomes, allowing for more precise drug administration to specific organs. Since nanoliposomes have an amphipathic shape, antioxidants contained inside these lipid carriers can inhibit the start of oxidation at food's water/oil interface (46). Studies by Lü et al. on the antioxidation status, signal transduction pathways, vascular system, and ligand-receptor interaction of ginseng extract demonstrated the efficacy of the active chemicals in ginseng, notably ginsenosides and their pharmaceutical uses (47). Nah et al. indicated that ginseng's bioactive ingredient, ginsenosides, had a favorable correlation with central and peripheral nervous system function (48).
Furthermore, green tea has unique chemical bioactive agents such as anti-inflammatory components, health-developing qualities, and high redox efficiency in medicinal herbal categorization (28). Also, there is mounting strong indication that curcumin is an effective natural compound with versatile therapeutic prospects in the treatment of neurodegenerative, cardiovascular, cancers, inflammation, infection, rheumatism, allergic, cell differentiation, and cell degeneration due to its anti-inflammatory activity and antioxidative effects (49). Potential pharmacological applications of M. incana with cytotoxic, enzyme inhibitory, and antioxidant properties were noted in some metabolic disorders like obesity or type 2 diabetes and different tumors like colorectal adenocarcinoma and breast cancer, as reported by Taviano et al. Furthermore, the extract of Ma. incana functions as an enzyme inhibitor in neuroprotection (50). Thus, we hypothesized that natural bioactive substances like ginseng, M. incana, turmeric, and green tea might significantly affect the protein-protein interaction network associated with midbrain tumors and movement instability.
Antioxidant, anti-inflammatory, antibacterial, antiviral, antiallergenic, and antithrombotic properties are only a few of phenolic compounds' various biological impacts (51). While directly used bioactive phenolic chemicals can negatively impact enzymatic activity in the digestive tract, the phenolic chemical compounds can destroy during digestion (46). As a result, liposomes were developed as a flexible assembly to circumvent the restrictions placed on the use of herbal extracts in treating diseases; liposomes are effective carriers for the delivery of herbal extracts with more therapeutic advantages (52). Liposomes can potentially treat illnesses and improve signatures as effective carriers of herbal extracts due to biodegradable criteria and drug transport capabilities in para- and transcellular directions (53).
Hence, we explored that the swimming training and nanoliposomes enriched combined supplement ameliorated the inflammasome activation in the brain tumor rat models. Based on our data, this study indicated that physical activity and nanoliposomes enriched combined supplement could improve the inflammasome activation tumor suppressor, cell division, and nerve function. Moreover, in silico analysis identified that the p53, Hras, IL-10, and Casp8 hub genes could consider biomarkers for prognosis and diagnosis in the brain tumor. Furthermore, targeting P53, Hras, IL-10, and Casp8 networks might improve the quality of life and reduce inflammation activity and oxidative stress.
![Swimming training and nanoliposomes enriched combined supplement improved gene expressions. a-d: the relative expression of IL-10, Casp8, P53, and Hras [data expressed as mean ± SD. + indicates statistically significant difference with normal group, * indicates statistically significant difference with Model group, @ indicates statistically significant difference with Model + Liposome group, # indicates statistically significant difference with Model + EXE group, & indicates statistically significant difference with model + extract group, ! indicates statistically significant difference with model + liposomal extract group, ^ indicates statistically significant difference with model + exe + extract group, $ indicates statistically significant difference with model + exe + liposomal extract. The data were calculated by one-way analysis of variance (ANOVA) with Tukey's post hoc test. The effects of the intervention and the interaction effects of the interventions were detected based on the two-way analysis of variance (ANOVA) with Tukey's post hoc test due to multiple comparisons]. Swimming training and nanoliposomes enriched combined supplement improved gene expressions. a-d: the relative expression of IL-10, Casp8, P53, and Hras [data expressed as mean ± SD. + indicates statistically significant difference with normal group, * indicates statistically significant difference with Model group, @ indicates statistically significant difference with Model + Liposome group, # indicates statistically significant difference with Model + EXE group, & indicates statistically significant difference with model + extract group, ! indicates statistically significant difference with model + liposomal extract group, ^ indicates statistically significant difference with model + exe + extract group, $ indicates statistically significant difference with model + exe + liposomal extract. The data were calculated by one-way analysis of variance (ANOVA) with Tukey's post hoc test. The effects of the intervention and the interaction effects of the interventions were detected based on the two-way analysis of variance (ANOVA) with Tukey's post hoc test due to multiple comparisons].](https://services.brieflands.com/cdn/serve/3170b/8fd0f9fc58c79441f372499e68ad9d54a3ad2c11/mcj-131461-i003-F3-preview.webp)
![The effect of swimming training and nanoliposomes enriched combined supplement on the behavior tests [data are expressed as mean ± SD. + indicates statistically significant difference with Normal group, * indicates statistically significant difference with model group, @ indicates statistically significant difference with model + liposome group, # indicates statistically significant difference with model + exe group, & indicates statistically significant difference with model + extract group, ! indicates statistically significant difference with model + liposomal extract group, ^ indicates statistically significant difference with model + exe + extract group, $ indicates statistically significant difference with model + exe + liposomal Extract. The data were calculated by one-way analysis of variance (ANOVA) with Tukey's post hoc test. The effects of the intervention and the interaction effects of the interventions were detected based on the two-way analysis of variance (ANOVA) with Tukey's post hoc test due to multiple comparisons]. The effect of swimming training and nanoliposomes enriched combined supplement on the behavior tests [data are expressed as mean ± SD. + indicates statistically significant difference with Normal group, * indicates statistically significant difference with model group, @ indicates statistically significant difference with model + liposome group, # indicates statistically significant difference with model + exe group, & indicates statistically significant difference with model + extract group, ! indicates statistically significant difference with model + liposomal extract group, ^ indicates statistically significant difference with model + exe + extract group, $ indicates statistically significant difference with model + exe + liposomal Extract. The data were calculated by one-way analysis of variance (ANOVA) with Tukey's post hoc test. The effects of the intervention and the interaction effects of the interventions were detected based on the two-way analysis of variance (ANOVA) with Tukey's post hoc test due to multiple comparisons].](https://services.brieflands.com/cdn/serve/3170b/cea58b3501331b265f0007af181356bd316b5e15/mcj-131461-i004-F4-preview.webp)