1. Background
Infections associated with healthcare services are among the common adverse effects that could happen in hospitalized patients. They are also an important public health issue that causes an increase in morbidity and mortality and has negative effects on the quality of life of the public (1). These infections, which are also referred to as nosocomial infections, cause an important economic burden socially. On the other hand, an important percentage of them could be prevented using effective infection prevention and control precautions. Hospital-related infections spread not only among patients but also among health professionals (1).
In extraordinary cases like pandemics, healthcare professionals are expected to provide several medical procedures requiring close contact with individuals who have an infectious disease, sometimes under intense working conditions and sometimes with inadequate resources and for long working hours (2). Providing care to individuals with a highly infectious disease increases the potential infection from patients to healthcare professionals (3).
The new coronavirus disease, which is a highly contagious disease that emerged from China, has caused a great burden on the healthcare services of all countries; it has caused numerous healthcare professionals who provided services to these patients to be infected with the virus and lose their lives (4). This global pandemic was declared a “public health emergency of international concern” by the World Health Organization in January 2020 (5).
The greatest risk in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is reported to be direct unprotected contact or direct contact with individuals with the disease and exposure to respiratory droplets during this contact (6). This infection continues to be a serious threat, particularly to healthcare professionals. The infection rates of SARS-CoV-2 among healthcare professionals are reported to range from 0.4% to 57.1% (7, 8).
This disease, which is also reported to be transmitted in a nosocomial way, is transmitted to healthcare professionals due to factors, such as ignoring the use of masks, removing masks during break times and meals, and failing to maintain physical distance (9). Patients who have SARS-CoV-2 demonstrate severe respiratory symptoms. They could also develop other complications, such as shock, acute renal damage, and gastrointestinal hemorrhaging. Therefore, approximately 5% to 30% of patients with SARS-CoV-2 infections require intensive care monitoring and ventilation support (10-12).
Healthcare professionals could acquire nosocomial infections due to factors, such as failing to apply infection control precautions during the implementation of medical procedures, the production of aerosols, such as intubation, aspiration, invasive and noninvasive ventilation, and nebulization for the treatment of patients in intensive care units (ICUs) (11, 13-15).
Isolation precautions, which have an important role in controlling hospital-related infections, include forming a barrier to prevent the transmission of resistant microorganisms and infectious diseases to patients, healthcare professionals, and visitors (1). Effective isolation can be maintained by putting patients in single rooms, providing them with clear and comprehensible information about their disease, isolating the equipment used for these patients, using protective equipment, and following standard precautions by healthcare professionals (1).
While entering the room of a patient with a suspected or confirmed SARS-CoV-2 infection, health professionals are recommended to follow standardized precautions and use an N95 equivalent or higher-level mask, glasses and face shield, nonsterile gloves, and an isolation gown (3). It is highly important for healthcare professionals to carefully follow infection control precautions for decreasing or preventing SARS-CoV-2 transmissions among health units to both protect themselves and prevent health service-related transmissions. Healthcare professionals’ attitudes toward isolation precautions need to be evaluated periodically, particularly in units, such as ICUs, which have a higher workload and medical implementations with higher risks in terms of transmission (16-18).
The ICUs are places where the treatment and care of patients requiring long-term hospitalization are conducted; invasive interventions are frequently implemented; therefore, there is a higher prevalence of health service-related infections for healthcare professionals (19-21). Isolation precautions have an important place in controlling health service-related infections, and it is recommended to periodically assess the knowledge and attitudes of healthcare professionals regarding this issue (1, 17, 18, 22).
2. Objectives
This study aimed to identify the SARS-CoV-2 infection status and degree of compliance with and attitudes toward isolation precautions among doctors and nurses working in ICUs.
3. Methods
3.1. Design and Setting
This cross-sectional study was conducted in the ICUs of the Education and Research Hospital, University Research Hospital, and City Hospital in eastern Turkey within March to May 2021.
3.2. Sample
The target population of this study included 447 doctors and nurses who worked in the ICUs of the above-mentioned hospitals. The sample size was calculated to be 205 individuals who were selected using a sampling method with a known population (23, 24), and the participants were included in the sample using the convenience sampling method.
3.2.1. Inclusion Criteria
The study involved doctors and nurses who worked in ICUs and who were not on leave during the study time.
3.2.2. Exclusion Criteria
Those with the condition that is contraindicated from the use of protective equipment (e.g., latex allergy) were not included in the study.
3.3. Measures
The data were collected using the sociodemographic form and compliance with isolation precautions scale.
3.3.1. The Sociodemographic Form
The form consists of 29 items that were prepared by the researchers based on the related literature and investigated health professionals’ descriptive features and ascertained whether or not they had been involved in conditions that might pose a risk in terms of infection transmission (25).
3.3.2. Compliance with Isolation Precautions Scale
The scale was developed by Tayran and Ulupınar in 2010 (26). It is a 5-point scale with 18 items, 14 positive and 4 negative items, which indicates the degrees of compliance with isolation precautions. Cronbach’s alpha was observed to be 0.85 for the total scale, and the explained variance ratio was reported to be 50.50% (26). Higher scale scores indicate increased compliance with isolation precautions. Cronbach’s alpha was reported to be 0.88 in this study.
3.4. Data Collection
After being informed about the purpose of the study, the doctors and nurses who agreed to participate in the study were administered the data collection scales by two researchers who were infectious diseases specialists and one ICU nurse in the hospitals where the study was conducted. A link was created to have access to the questions prepared online via Google forms. The link was sent to the participants via telephone so that they could respond to the questions.
3.5. Analysis
The data were analyzed in SPSS software (version 18) using descriptive statistics to analyze the groups by their descriptive features. The Kolmogorov-Smirnov test was used to determine whether the data set was normally distributed; as the data were not normally distributed, the Mann-Whitney U test was utilized to compare mean scores in two independent groups. The Kruskal-Wallis test was applied to compare mean scores in more than two independent groups. The chi-square relationship test was used to examine the relationship between two categorical variables. Statistical significance was accepted at P < 0.05.
3.6. Ethical Considerations
This study was performed in line with the principles of the Declaration of Helsinki. Before the study, approval was obtained from the University Scientific Research Ethics Committee. Written permissions were obtained from the Ministry of Health of the Republic. Permission to use the scale was obtained from the corresponding author of the original scale. Written and verbal permissions were obtained from the nurses and doctors after being informed about the study.
4. Results
The participants’ demographic characteristics showed that their average age was 29.95 ± 7.16 years. Moreover, 77.6% of the participants were nurses. In this study, 40% of the participants were diagnosed with SARS-CoV-2, and all of them considered the hospital environment as the source of infection. Furthermore, 62% of the subjects did not experience inadequacy in equipment supply. The mean score of the compliance with isolation precautions scale was 77.53 ± 9.28 (Table 1).
| Categorical Variables | Variables | Values |
|---|---|---|
| Gender | Female | 136 (66.3) |
| Male | 69 (33.7) | |
| Profession | Doctor | 46 (22.4) |
| Nurse | 159 (77.6) | |
| Working unit | Surgical ICU | 43 (21.0) |
| Internal diseases ICU | 93 (45.4) | |
| Anesthesiology-reanimation | 28 (13.7) | |
| Neonatal-pediatrics ICU | 41 (20.0) | |
| Having been diagnosed with SARS-CoV-2 | Yes | 82 (40.0) |
| No | 123 (60.0) | |
| Place regarded as the source of infection (n = 82) | ICU | 41 (20.0) |
| Other areas in the hospital | 41 (20.0) | |
| Inadequate equipment supplies during isolation processes | Yes | 78 (38.0) |
| No | 127 (62.0) | |
| Scale Variables | Minimum, Maximum | |
| Age (y) | 29.95 ± 7.16 | 21, 60 |
| Duration of working in ICU (y) | 5.21 ± 5.74 | 1 (month), 36 |
| Duration of working with patients with suspected/confirmed SARS-CoV-2 | 6.24 ± 4.40 | 1, 24 |
| Compliance with isolation precautions scale total | 77.53 ± 9.28 | 38, 90 |
Abbreviations: ICU, intensive care unit; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
a Values are expressed as No. (%) unless otherwise indicated.
The results of the chi-square test that aimed to identify the relationship between receiving a SARS-CoV-2 infection diagnosis and high-risk procedures (i.e., tracheal intubation or removing the tracheal tube, tracheostomy, cardiopulmonary resuscitation, subglottic secretion aspiration, fiberoptic bronchoscopy or laryngoscope procedure, providing the mechanic ventilator support, and providing routine care) used for the patients indicated no relationships between the variables (Table 2).
| Had Diagnosis | Did Not Have the Diagnosis | P-Value | |
|---|---|---|---|
| I performed tracheal intubation/I accompanied the practice | 0.384 | ||
| Yes | 74 (90.2) | 106 (86.2) | |
| No | 8 (9.8) | 17 (13.8) | |
| I performed a tracheostomy/I was present at the time of the procedure | 0.954 | ||
| Yes | 35 (42.7) | 52 (42.3) | |
| No | 47 (57.3) | 71 (57.7) | |
| I removed a tracheal tube/I was present at the time of the procedure | 0.090 | ||
| Yes | 70 (85.4) | 93 (75.6) | |
| No | 12 (14.6) | 30 (24.4) | |
| I performed cardiopulmonary resuscitation/I was present at the time of the procedure | 0.510 | ||
| Yes | 75 (91.5) | 109 (88.6) | |
| No | 7 (8.5) | 14 (11.4) | |
| I aspirated the patient’s subglottic secretion/I was present at the time of the procedure | 0.266 | ||
| Yes | 80 (97.6) | 116 (94.3) | |
| No | 2 (2.4) | 7 (5.7) | |
| I performed a fiberoptic bronchoscopy/I was present at the time of the procedure | 0.130 | ||
| Yes | 10 (12.2) | 25 (20.3) | |
| No | 72 (87.8) | 98 (79.7) | |
| I performed a laryngoscope procedure/I was present at the time of the procedure | 0.169 | ||
| Yes | 57 (69.5) | 96 (78.0) | |
| No | 25 (30.5) | 27 (22.0) | |
| I provided the patient with a mechanic ventilator support/I was present at the time of the procedure | 0.366 | ||
| Yes | 78 (95.1) | 113 (91.9) | |
| No | 4 (4.9) | 10 (8.1) | |
| I provided the patient with routine care/I was present at the time of the procedure | 0.483 | ||
| Yes | 78 (95.1) | 114 (92.7) | |
| No | 4 (4.9) | 9 (7.3) |
a Values are expressed as No. (%).
The results of the chi-square test indicated a significant relationship between the diagnosis of SARS-CoV-2 infection and the diagnosis of other individuals living in the same house (P < 0.001). Posthoc analysis (Cramer’s V test) showed that there was a moderate-level relationship between SARS-CoV-2 infection diagnosis and the diagnosis of other individuals living in the same house (r = 0.423, P < 0.001). No relationships were observed between other variables (i.e., hand hygiene, removing masks in common rest areas, duration of working with SARS-CoV-2 patients, and compliance with isolation precautions scale’s mean score) and diagnosis status (Table 3). The results of the chi-square test, which was conducted to identify the relationship between ICU healthcare professionals’ having a diagnosis of SARS-CoV-2 and using protective equipment during close contact with SARS-CoV-2 patients with or without a mask, indicated no relationship between the variables (Table 4).
| Had Diagnosis | Did Not Have the Diagnosis | P-Value | |
|---|---|---|---|
| I could not wash my hands as recommended | 0.928 | ||
| Yes | 9 (11.0) | 14 (11.4) | |
| No | 73 (89.0) | 109 (88.6) | |
| I used personal protective equipment wrongly | 0.588 | ||
| Yes | 8 (9.8) | 15 (12.2) | |
| No | 74 (90.2) | 108 (87.8) | |
| I removed my mask in common rest places while there were others | 0.904 | ||
| Yes | 54 (65.9) | 82 (66.7) | |
| No | 28 (34.1) | 41 (33.3) | |
| We consumed food in the same environment with other individuals who worked in the same unit | 0.060 | ||
| Yes | 75 (91.5) | 101 (82.1) | |
| No | 7 (8.5) | 22 (17.9) | |
| We consumed food in the same environment with other individuals who worked in a different unit | 0.145 | ||
| Yes | 22 (26.8) | 45 (36.6) | |
| No | 60 (73.2) | 78 (63.4) | |
| Showing high-risk behaviors apart from the ones indicated (e.g., participating in social activities and using public transportation) | 0.832 | ||
| Yes | 6 (7.3) | 10 (8.1) | |
| No | 76 (92.7) | 113 (91.9) | |
| Experiencing inadequate equipment supplies of personal protective equipment | 0.436 | ||
| Yes | 31 (37.8) | 40 (32.5) | |
| No | 51 (62.2) | 83 (67.5) | |
| SARS-CoV-2 diagnosis of individuals sharing the same house | 0.001 | ||
| Had | 50 (61.0) | 24 (19.5) | |
| Did not have | 32 (39.0) | 99 (80.5) | |
| Duration of working with individuals who had suspected or confirmed SARS-CoV-2 (mo) | 6.01 ± 4.16 | 6.40 ± 4.57 | 0.725 |
| Compliance with isolation precautions scale total | 77.85 ± 9.18 | 77.32 ± 9.38 | 0.650 |
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
a Values are expressed as No. (%) or
| Had Diagnosis | Did Not Have the Diagnosis | P-Value | |
|---|---|---|---|
| During Close Contact with a SARS-CoV-2 Patient Without a Mask | |||
| There were times when I did not use a mask | 1.00 | ||
| Yes | 8 (9.8) | 12 (9.8) | |
| No | 74 (90.2) | 111 (90.2) | |
| There were times when I used a medical mask in cases of N95 indication | 0.863 | ||
| Yes | 37 (45.1) | 54 (43.9) | |
| No | 45 (54.9) | 69 (56.1) | |
| There were times when I did not use an eye protector | 0.457 | ||
| Yes | 35 (42.7) | 59 (48.0) | |
| No | 47 (57.3) | 64 (52.0) | |
| There were times when I did not use gloves and gowns | 0.806 | ||
| Yes | 11 (13.4) | 18 (14.6) | |
| No | 71 (86.6) | 105 (85.4) | |
| During Close Contact with a SARS-CoV-2 Patient with a Mask | |||
| There were times when I did not use a mask, or I used a medical mask in cases of N95 indication | 0.953 | ||
| Yes | 29 (35.4) | 44 (35.8) | |
| No | 53 (64.6) | 79 (64.2) | |
| There were times when I did not use an eye protector | 0.304 | ||
| Yes | 36 (43.9) | 63 (51.2) | |
| No | 46 (56.1) | 60 (48.8) | |
| There were times when I did not use gloves and gowns | 0.753 | ||
| Yes | 12 (14.6) | 20 (16.3) | |
| No | 70 (85.4) | 103 (83.7) | |
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
a Values are expressed as No. (%).
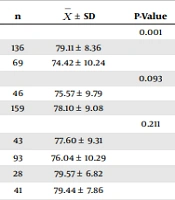
When the scale’s mean scores of the intensive care professionals were analyzed, it was found that the scale’s mean score was significantly higher in those who were female, maintained hand hygiene as recommended before contact with the patients, used personal protective equipment correctly, and always used masks, eye protectors, gloves, and gowns while in contact with SARS-CoV-2 patients with or without a mask, and the difference was statistically significant (P < 0.05) (Table 5).
| n | P-Value | ||
|---|---|---|---|
| Gender | 0.001 | ||
| Female | 136 | 79.11 ± 8.36 | |
| Male | 69 | 74.42 ± 10.24 | |
| Profession | 0.093 | ||
| Doctor | 46 | 75.57 ± 9.79 | |
| Nurse | 159 | 78.10 ± 9.08 | |
| Working unit | 0.211 | ||
| Surgical ICU | 43 | 77.60 ± 9.31 | |
| Internal diseases ICU | 93 | 76.04 ± 10.29 | |
| Anesthesiology-reanimation | 28 | 79.57 ± 6.82 | |
| Neonatal-pediatrics ICU | 41 | 79.44 ± 7.86 | |
| Having SARS-CoV-2 diagnosis | 0.650 | ||
| Yes | 82 | 77.85 ± 9.18 | |
| No | 123 | 77.32 ± 9.38 | |
| I could not wash my hands as recommended | 0.001 | ||
| Yes | 23 | 70.61 ± 10.75 | |
| No | 182 | 78.41 ± 8.73 | |
| I could not maintain hand hygiene sufficiently before contacting patients | 0.002 | ||
| Yes | 21 | 70.95 ± 11.51 | |
| No | 184 | 78.28 ± 8.72 | |
| I could not maintain hand hygiene sufficiently after contacting patients | 0.073 | ||
| Yes | 16 | 73.25 ± 10.52 | |
| No | 189 | 77.89 ± 9.11 | |
| I used personal protective equipment wrongly | 0.023 | ||
| Yes | 23 | 74.35 ± 8.25 | |
| No | 182 | 77.93 ± 9.35 | |
| During Close Contact with a SARS-CoV-2 Patient Without a Mask | |||
| There were times when I did not use a mask | 0.001 | ||
| Yes | 20 | 70.40 ± 11.27 | |
| No | 185 | 78.30 ± 8.73 | |
| There were times when I used a medical mask in cases of N95 indication | 0.138 | ||
| Yes | 91 | 76.81 ± 8.87 | |
| No | 114 | 78.11 ± 9.60 | |
| There were times when I did not use an eye protector | 0.014 | ||
| Yes | 94 | 75.68 ± 10.19 | |
| No | 111 | 79.10 ± 8.16 | |
| There were times when I did not use gloves and gowns | 0.001 | ||
| Yes | 29 | 68.93 ± 12.42 | |
| No | 176 | 78.95 ± 7.84 | |
| During Close Contact with a SARS-CoV-2 Patient with a Mask | |||
| There were times when I did not use a mask, or I used a medical mask in cases of N95 indication | 0.004 | ||
| Yes | 73 | 75.30 ± 9.64 | |
| No | 132 | 78.77 ± 8.87 | |
| There were times when I did not use an eye protector | 0.011 | ||
| Yes | 99 | 76.33 ± 8.62 | |
| No | 106 | 78.65 ± 9.77 | |
| There were times when I did not use gloves and gowns | 0.001 | ||
| Yes | 32 | 71.09 ± 11.62 | |
| No | 173 | 78.72 ± 8.29 | |
Abbreviations: ICU, intensive care unit; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
5. Discussion
This study aimed to identify the SARS-CoV-2 infection status and compliance with and attitudes toward isolation precautions among doctors and nurses working in ICUs during the pandemic. The results of this study showed that doctors and nurses displayed good compliance with isolation precautions (the scale mean score: 77.53 ± 9.28). Moreover, 40% of the ICU healthcare professionals had a SARS-CoV-2 infection diagnosis, and a significant relationship was observed between this diagnosis and the diagnosis of other individuals who shared the same house (P < 0.001).
The compliance with isolation precautions scale’s mean scores of the participating doctors and nurses were observed to be 75.57 ± 9.79 and 78.10 ± 9.08, respectively, indicating a good level of compliance with isolation precautions. Studies in the literature similarly reported good levels of scale mean scores among doctors and nurses (26-28). In a study conducted on nurses, Karahan et al. reported good levels of scale’s mean scores (29). Although studies conducted in Turkey reported similar results, some studies conducted in different countries reported lower isolation compatibility.
Suliman et al. reported that nurses had a good knowledge level of isolation and indicated that compatibility with isolation was not sufficient. They noted that barriers to isolation were associated with high workloads and a lack of equipment (30). Moriceau et al. reported that doctors had insufficient knowledge about isolation precautions (31). Differences in the study results are considered to be caused by the healthcare system and policies in the countries where the studies were conducted. Compliance with isolation precautions is reported to be important in controlling the spread of multiresistant microorganisms and diseases associated with them (32-34). Participating professionals’ good compliance level of isolation precautions is important in terms of enhancing infection control in ICUs.
In the present study, 40% of the participants were diagnosed with SARS-CoV-2 (Table 1). Wong et al. reported that the infection rate was within the range of 2.1 - 29% among health professionals in China (7). A meta-analysis that evaluated the prevalence and risk factors among health professionals investigated the results of 46 studies, including 31 studies in Europe, 9 studies in America, and 6 studies in Asia. The researchers in the aforementioned studies evaluated the prevalence of SARS-CoV-2 infections among healthcare professionals using reverse transcription-polymerase chain reaction (PCR). The meta-analysis results showed that the prevalence of SARS-CoV-2 infection ranged from 0.4% to 57.1% (8).
No relationships were observed between doctors’ and nurses’ SARS-CoV-22 diagnosis with implementing procedures that produced aerosol in patients, using protective equipment during close contact with SARS-CoV-2 patients with or without a mask, the duration of working with these patients, hand hygiene behaviors, and removing masks in common rest areas (Tables 2 and 3). The literature involves studies reporting that inadequate hand hygiene, use of medical masks instead of N95, lack of personal protective equipment or not using it, removing masks during eating and drinking in rest areas, and failing to protect physical distances increased the SARS-CoV-2 risk among healthcare professionals (9, 25, 35, 36). A multicentered cohort study that investigated whether the implementation of procedures that produced aerosols posed a risk to healthcare professionals reported that SARS-CoV-2 incidence increased among health professionals performing tracheal intubation; however, no causal relationship was reported between tracheal intubation and SARS-CoV-2 results (37).
In addition, Folgueira et al. compared positive SARS-CoV-2 rates among healthcare professionals according to the risk level in the units in which they worked. They found that SARS-CoV-2 positive PCR rates indicated no statistically significant differences among healthcare professionals who worked in units where they were in close contact with SARS-CoV-2 patients and those who worked in high-risk areas in terms of high aerosol production in comparison to those who worked in units that had a medium to low risk (38). In their study that also investigated genomic diversity, Sikkema et al. reported that healthcare professionals commonly had community-acquired infections rather than nosocomial infections (39). Moreover, approximately half of the healthcare professionals who were infected with SARS-CoV-2 were asymptomatic during screenings and reported that infection could happen from asymptomatic carriers to other healthcare professionals (8). Although some of the findings obtained in this study are similar to the findings in the literature, some others demonstrated differences. This difference is considered to possibly result from health systems, policies, management of the pandemic process, and individual factors in the study groups. There is a need for prospective studies with a high evidence level conducting an in-depth investigation of the risk factors associated with infections of healthcare professionals.
A moderate-level significant relationship was observed between the diagnosis of ICU professionals and the diagnosis of individuals who shared the same house (P < 0.01) (Table 3). Closed areas and close distances are reported to increase the risk of SARS-CoV-2 (40). Therefore, Qian et al. reported a spread among individuals sharing the same house (41). In addition, some studies provided evidence demonstrating that transmission to healthcare professionals could be community-acquired (39, 42, 43). A meta-analysis investigating these studies indicated that in-house contact could play an important role in SARS-CoV-2 infections among healthcare professionals due to the rapid spread of the virus in the community (8).
The results of this study showed that gender had an effect on compliance with isolation, and the scale’s mean score was significantly higher in women (P < 0.05) (Table 5). Although the literature includes studies demonstrating that compliance was better in the female gender (44, 45), there are also studies indicating that gender did not affect compliance with isolation (27-29).
The compliance with isolation precautions scale’s mean score was observed to be significantly higher in those who maintained hand hygiene sufficiently as recommended and before making contact with the patient, in those who used personal protective equipment correctly, and in those who always used masks, eye protection, gloves, and gowns while in contact with SARS-CoV-2 patients with or without a mask, and the difference between the groups was observed to be significantly higher (P < 0.05) (Table 5). Some studies show that nurses’ attitudes toward isolation were not at a sufficient level, and therefore compliance with isolation was insufficient (46, 47). A study conducted by Mohd-Nor and Bit-Lian on ICU nurses reported that attitudes toward and compliance with isolation precautions were good and highlighted that correct attitudes increased these practices (48). Studies conducted on student nurses showed a significant relationship between attitudes toward isolation precautions and compliance (44, 49). A study conducted on postgraduate trainee doctors reported that they had a moderate-level awareness about the components of standard precautions for SARS-CoV-2 and that this condition could cause inadequate practices (50).
Providing healthcare professionals with training about the use of personal protective equipment and isolation precautions by infection control units increases their knowledge level. It is important to remember that compliance could be increased, and correct attitudes could be adopted by controlling factors that prevent healthcare professionals’ compliance with isolation (e.g., the inadequacy of equipment supply and heavy workload). Administrators in health institutions should consider this information while making plans, particularly during pandemics, to control the pandemic.
5.1. Conclusions
The results of this study showed that compliance with isolation was observed to be better in those who maintained hand hygiene sufficiently and accurately, used protective equipment correctly, and always used masks, eye protection, gloves, and gowns during close contact with SARS-CoV-2 patients. A significant relationship was observed between ICU professionals with a SARS-CoV-2 infection diagnosis and the diagnosis of individuals who shared the same house. In-depth studies of SARS-CoV-2 infection risk factors among doctors and nurses working in ICUs might be the focus of future research.
5.2. Limitations
The limitation of this study is that behaviors indicating compliance with the isolation of the ICU professionals were not monitored by an observer or observers, and the participants were included in the sample using the convenience sampling method.