1. Background
Celiac disease (CD), also known as celiac sprue and gluten-sensitive enteropathy, is a common autoimmune disease in genetically predisposed individuals through the gluten protein in rye and wheat. Although this disease had been known as a pure gastrointestinal disease, it is now considered an inflammatory disease of the gastrointestinal tract that affects the small intestine, especially its initial parts (1). A systematic review of the global prevalence of CD demonstrated a seroprevalence rate of 1.4%, with the prevalence varying by continent, ranging from 1.3 (South America) to 1.8% (Europe and Asia) (2). Furthermore, based on the literature, its prevalence is about 1% in the general population of Iran (3-5). This disease is more common in childhood and adolescence but also occurs in adulthood. Approximately 20% of patients diagnosed with this disease are over 60 years old (6). Asymptomatic CD can progress to classic or typical intestinal symptoms such as diarrhea, weight loss, and abdominal pain, as well as atypical or nonclassical symptoms such as iron deficiency, bloating, constipation, chronic fatigue, headache, osteoporosis, neurologic disorders (e.g., depression and gluten ataxia), reproductive disorders (e.g., menarche and menopausal disorders), and oral/cutaneous disorders (e.g., dermatitis) (7-10).
Numerous studies in Iran have examined the symptoms of CD and related diseases. For example, Ganji et al. reported that the classic type of CD was the most common in northeast Iran. Moreover, female CD patients are more likely to have concomitant disorders such as nervous problems, bone diseases, and anemia (11). Dehbozorgi et al. reported that the patients' most common gastrointestinal symptoms were abdominal pain, flatulence, and constipation. Furthermore, the most common extraintestinal manifestations included bone pain, long-term fatigue, and anemia. The most frequent comorbidities associated with CD in children were type 1 diabetes mellitus and hypothyroidism (12).
2. Objectives
Because CD is relatively standard and associated with other diseases, collecting data on its prevalence and associated factors can assist authorities in planning early diagnostic and therapeutic measures. Therefore, the present study aimed to determine the epidemiological characteristics, clinical symptoms, and laboratory findings of people with CD in South Khorasan Province, Iran.
3. Methods
3.1. Study Design
This descriptive study was conducted on all patients with new cases of CD who were referred to the Gastrointestinal Clinic in South Khorasan from March to August 2019.
3.2. Participants
In total, 110 individuals with CD participated in this study. After obtaining written informed consent from all participants, their medical history and medication adherence information were collected using a questionnaire. A checklist was also administered to collect demographic information, medical records, family history of the disease, symptoms, laboratory diagnostic tests (evaluation of anti-tissue transglutaminase immunoglobulin A (anti-TTG-IgA levels)), and biopsy results using the Marsh classification.
3.3. Scales
Histological examinations were performed for a definitive diagnosis of CD based on the Marsh classification. In Marsh I, the natural appearance of the mucosa was accompanied by an increase in lymphocytes inside the epithelium. In Marsh II, the height of the intestinal villi was shortened, which was attributed to hyperplastic crypts. Marsh III is associated with hyperplastic crypts and a moderate-to-severe reduction of villi. Most patients with celiac disease are classified as Marsh III at diagnosis (13, 14).
3.4. Data Collection
Histological examinations were performed for a definitive diagnosis of CD based on the Marsh classification. In Marsh I, the natural appearance of the mucous is accompanied by an increase in lymphocytes inside the epithelium. In Marsh II, the height of the intestinal villi is shortened, which is attributed to hyperplastic crypts. Marsh III is associated with hyperplastic crypts and a moderate-to-severe reduction of villi. Most celiac patients were classified as Marsh III at diagnosis (13, 14).
3.5. Data Analysis
The data were used for epidemiological inquiry, and the corresponding analyses were performed using SPSS v.22.
3.6. Ethical Consideration
This study was approved by the Research Council and Ethics Committee of Birjand University of Medical Science (ethics code: IR. BUMS. REC.1398.285). Informed written consent was obtained from all patients. No cost was imposed on the patients. The final report and analysis were performed without the names of the study participants.
4. Results
4.1. Demographic Information
We aimed to determine the frequency distribution of clinical and demographic characteristics of 110 individuals with CD in South Khorasan from March to August 2019. The population consisted of 78 (70.9%) men and 32 (29.1%) women, with a mean age of 15.25 ± 28.38 years (median = 27.5 and IQR = 24), ranging from 4 to 71 years.
The findings regarding gastrointestinal symptoms showed that abdominal pain in 70 (63.6%) and diarrhea in 44 (40%) were the most common symptoms among the patients (Table 1). Regarding nongastrointestinal symptoms, 22 (20%) individuals reported skin problems (dermatitis herpetiformis), 15 (13.6%) had oral problems (mouth ulcers), and 10 (9.1%) noted bone problems (osteoporosis and osteopenia).
| Clinical Symptoms | No. (%) |
|---|---|
| Abdominal pain | 70 (63.6) |
| Constipation | 43 (39.1) |
| Diarrhea | 44 (40) |
| Nausea | 35 (31.8) |
| Skin disorders (dermatitis herpetiformis) | 22 (20) |
| Oral disorders (mouth ulcers) | 15 (13.6) |
| Bone disorders (osteoporosis and osteopenia) | 10 (9.1) |
The Frequency Distribution of Clinical Gastrointestinal and Nongastrointestinal Symptoms in the Participants
4.2. Laboratory Findings
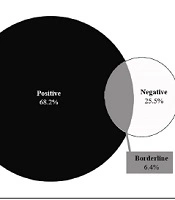
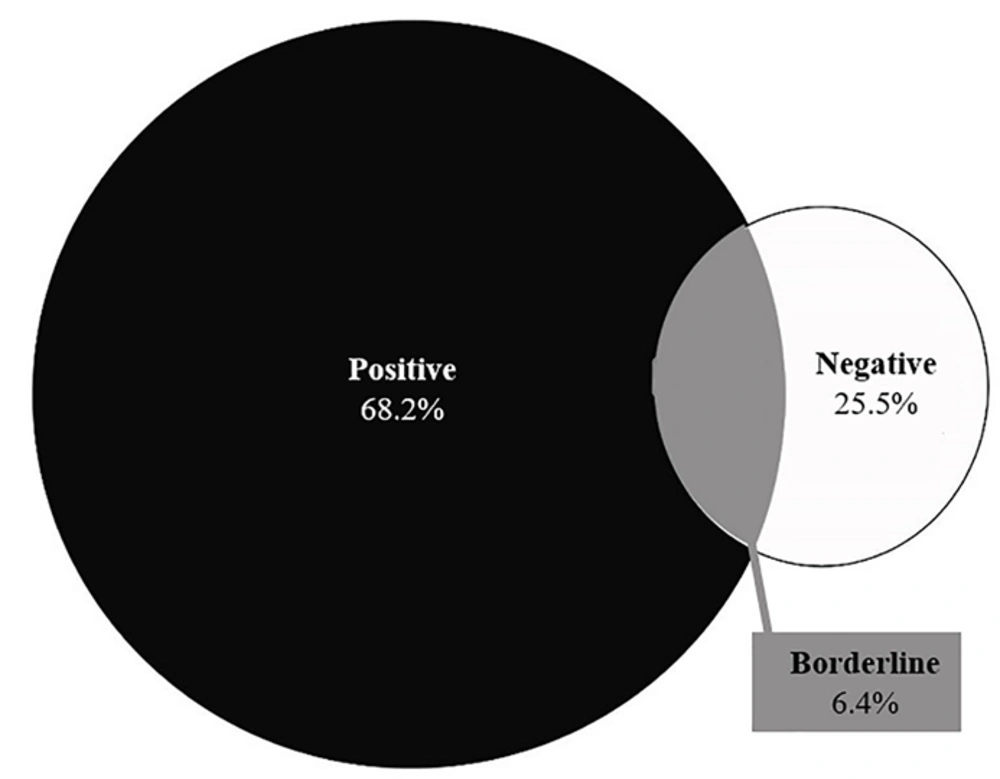
The frequency distribution of serum anti-tissue transglutaminase IgA (anti-TTG-IgA) levels revealed that immunoglobulin levels were less than 12 U/mL (negative) in 28 (25.5%), between 12 and 18 U/mL (borderline) in 7 (6.4%), and greater than 19 (positive) in 75 (68.2%) patients (Figure 1).
4.3. Diagnostic and Interventional Results
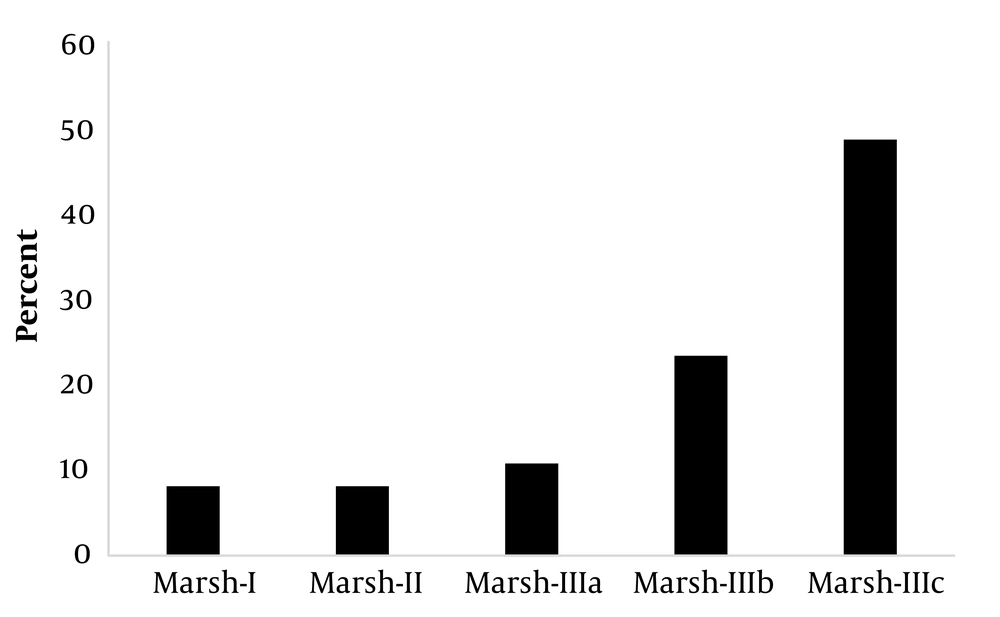
The diagnostic and interventional findings showed that all the participants underwent upper gastrointestinal endoscopy, but only two had colonoscopies. The results of gastrointestinal biopsy pathology among those who underwent endoscopy revealed that most participants were in the Marsh 3c (n = 54, 49.1%) and Marsh 3b (n = 26, 23.6%) categories (Figure 2).
4.4. Frequency of Comorbidities in Patients with Celiac Disease
Of 110 participants, 17 (15.5%) had musculoskeletal disorders, 17 (15.5%) had psychiatric problems, 11 (10%) had iron deficiency anemia, 10 (9.1%) had Sjogren's syndrome, and 8 (7.3%) had diabetes (Appendix 1 in the Supplementary File).
5. Discussion
Celiac disease was initially proposed to affect white Europeans exclusively. However, recent epidemiological studies have reported its occurrence in almost every part of the world, including Africa, the Middle East, Asia, and South America (13). The global prevalence of CD is approximately 0.5-1%, except for those who consume very little or no gluten (14). Celiac disease is a relatively common cause of chronic diarrhea in Iran, Iraq, and Kuwait and is diagnosed in 2-8% of patients with type 1 diabetes in Iran, Israel, and Saudi Arabia. These numbers are obtained while the per capita consumption of wheat in many of these countries is among the highest in the world (> 150 kg per person per year) (15). Due to the limited availability of epidemiological studies on the prevalence of CD and its symptoms in Iran, particularly in the country's southeast, this study looked into the frequency distribution of clinical and demographic characteristics of 110 CD patients in South Khorasan from March to August 2019. According to the findings, the most common symptoms in both sexes were abdominal pain and diarrhea. Previous research shows these symptoms affect 85 – 54% of the population (16). In a study by Rostami Nejad et al., the prevalence of diarrhea in CD patients ranged from 6.5 to 20% (17). Previous studies have also reported constipation as another common disease symptom, and women are more likely to suffer from it. The frequency of constipation in women may be affected by physiological differences in the anatomy of the pelvis, as well as other factors such as hormones, including estrogen and progesterone. One study found constipation in 52% of people, which was higher than ours (18). In an Italian study, constipation was found in 13% of the CD patients, which was lower than ours (19).
Celiac disease manifests as osteopenia, osteoporosis, arthritis, and inflammation (20). Our findings appear consistent with previous studies despite differences in populations and study areas. Regarding comorbidities, musculoskeletal problems, including osteoporosis (15.5%), psychological disorders such as depression (15.5%), and peripheral neuropathy (12.7%, were the most common diseases associated with CD. Most previous studies have found that CD increases the risk of depression and osteoporosis. A clinical visit should include a mood assessment. Besides, depression is prevalent in CD in varying degrees, ranging from 6 to 57%. This rate went from 6.5% (21) to 14% (22), 17% (23), and 19 - 24% (24).
A study by the US National Institutes of Health, which looked at the 12-month prevalence of major depressive episodes in American adults with CD, found that 6.7% of these patients showed some depression (25). Preventing osteoporosis in younger CD patients and aggressively treating this disease in older patients should also be prioritized. In women with CD, the risk of osteoporosis is more than twice that in men; this risk is also nearly 4 times higher in CD patients than in healthy people (26).
The first step in CD screening and diagnosis is evaluating serological markers and tTG-IgA antibodies. The second step is the detection of anti-endomysial antibodies (EMA). The sensitivity and specificity of both methods are high. Studies on diagnostic laboratory markers in patients indicated that anti-tTG-IgA had a sensitivity of 96.8%, a specificity of 91%, and a positive predictive value of 91.2%. In comparison, its negative predictive value and diagnostic accuracy were 96.8% and 97.7%, respectively (27). According to another study, the sensitivity of tTG-IgA and EMA tests was 92% for the diagnosis of CD, but the specificity of TTG and EMA tests was 98.5% and 100%, respectively (13).
A weak association was observed between lower tTG-IgA levels and intestinal enteropathy among symptomatic patients with CD (14). In contrast, tTG-IgA levels (> 100 U/mL) were found to be highly specific for Marsh III lesions (28). Our findings about the serum levels of anti-tTG-IgA in 110 participants showed that 28 (25.5%) had immunoglobulin levels of less than 12 U/mL (negative), 7 (6.4%) had immunoglobulin levels of around 12 - 18 U/mL (borderline), and 75 (68.2%) had immunoglobulin levels greater than or equal to 19 U/mL (positive). Their mean serum levels of anti-tTG-IgA were 16.352 ± 91.642 U/mL. Moreover, 9 (8.2%), 9 (8.2%), 12 (10.9%), 26 (23.6%), and 54 cases (49.1%) belonged to Marsh I, Marsh II, Marsh IIIa, Marsh IIIb, and Marsh IIIc classes, respectively. In a study of 49 participants, 3, 4, and 5 patients were categorized as having Marsh I, Marsh II, and Marsh IIIa, respectively (3, 28). Another study among 1440 people showed that 7 individuals had positive serological evidence for anti-tTG-IgA, 5 of whom were satisfied to undergo endoscopy, and all of them showed a Marsh IIIc classification of CD in their gastrointestinal biopsy (29). Singh et al. also reported Marsh 0 (n = 1), Marsh III (n = 8), Marsh II (n = 1), and Marsh IIIa (n = 1) classifications of CD among their participants (30). Tursi et al. studied 119 patients with CD and observed Marsh I lesions in 13 patients (10.92%), Marsh II in 24 anti-tTG (20.16%), Marsh IIIa in 27 anti-tTG (22.68%), Marsh IIIb in 31 anti-tTG (26.05%), and Marsh IIIc in 24 anti-tTG (20.16%) participants. This study revealed that the prevalence of anti-tTG and its mean serum values were higher in CD patients with severe enteropathy (Marsh IIIb-c lesions) than in those with mild enteropathy (Marsh I-3a) (31). Webb et al. noted that among 230 individuals with CD, 67 had low tTG-IgA levels (less than 5 U/mL), of whom 55% had Marsh III lesions (27). In a study by Karami, serological results revealed that 47% of the participants had tTG antibodies. Based on the disease severity classification, the highest frequency (57%) was linked to Marsh III. Furthermore, 10% of the CD patients were simultaneously diagnosed with diabetes (32).
Gastrointestinal pathology tests in the current study revealed that Marsh IIIc categorization is more common in CD patients, consistent with the findings of earlier studies (33). Tissue changes were also observed in people with CD, although 25.5% of the participants had harmful antibody levels. Factors such as different measurement methods and technical errors in the measurement process of this type of antibody can justify this finding. In this regard, false negatives caused by abnormal serum IgA levels, elimination of gluten from the diet, and even the intake of immunosuppressive drugs and corticosteroids should be considered (34).
5.1. Conclusions
Most of the patients in this study were male and within the age range of 10-20 years. Abdominal pain was considered the most common clinical symptom by the participants. All the pathologically examined patients showed evidence of CD, while approximately two-thirds were serologically positive.