1. Background
Vitamin D is a fat-soluble vitamin produced in the body when skin is exposed to sunlight, absorbed from diet and nutritional supplements, and metabolized in the liver and kidneys (1). Its main role is to maintain mineral and skeletal homeostasis. However, many studies have demonstrated an inverse relationship between vitamin D levels and the risk of common chronic diseases such as rheumatoid arthritis, liver failure, hypertension, and diabetes, as well as cardiovascular risk factors and mortality (2-4). Considering extracellular vitamin D roles in various physiological processes, including immunological functions, hormone secretion, and cell proliferation and differentiation, it has received particular attention lately (5). Low levels of this vitamin can also cause neuropsychiatric disorders such as depression, schizophrenia, Alzheimer's disease, and autism. In addition, epidemiological and clinical studies have demonstrated that individuals with low vitamin D levels are more vulnerable to depression (6, 7). In the past, vitamin D was known as an anti-rickets agent or the sunshine vitamin. In recent decades, decreased serum 25 hydroxyvitamin D (25 OH Vitamin D) levels have been attributed to insufficient milk consumption, lack of exposure to sunlight, and increased body mass index (1). Vitamin D deficiency is one of the most common nutrient deficiencies worldwide, with an estimated rate of 30 - 50% (25OH Vitamin D < 20 ng/mL), and is considered a global health problem (8).
It is estimated that more than half a billion people will be affected by type 2 diabetes by 2030. The main causes of type 2 diabetes are insulin resistance, pancreatic beta-cell dysfunction, and systemic inflammation (9). In insulin-sensitive organs such as muscle and adipose tissue, calcium is necessary for insulin-mediated intracellular processes. Moderate calcium consumption is necessary for the proper function of cells, nerves, and muscles. Therefore, vitamin D increases insulin sensitivity by stimulating and activating insulin receptors and regulates the metabolism of fatty acids in skeletal muscles and fat tissue, and contributes to calcium homeostasis (10, 11).
Diabetes is a common disease that can lead to kidney complications, eye problems such as retinal diseases (a disease related to the retina called retinopathy or retinal disease that causes blurred vision), high blood pressure, and peripheral nerve disorders. This disease also causes liver disorders (12). Research has shown that people with type 2 diabetes suffer more from liver diseases. This disease is a main cause of liver enzyme malfunction and other related liver diseases, including non-alcoholic fatty liver, cirrhosis, and liver failure (13, 14). In people with diabetes, the levels of transaminases slightly increase, which can be detected by biochemical tests such as aspartate aminotransferase and alanine aminotransferase and controlled (15). Consequently, liver diseases can lead to vitamin D deficiency, thus affecting liver functions and enzymes. Hence, the question arises whether vitamin D deficiency is the cause of liver dysfunction or is merely the result of liver injury (16). The nuclear vitamin D receptor mediates the biological effects of vitamin D. The expression of this nuclear receptor in skin tissue, immune cells, fat cells, non-parenchymal liver cells, and pancreatic beta cells demonstrates that vitamin D has other important physiological roles besides the homeostasis of mineral ions (17). Previous studies have demonstrated that vitamin D serum levels have an inverse relationship with insulin resistance, the risk of diabetes, and metabolic syndrome (18, 19). On the other hand, there is a relationship between liver enzyme levels and type 2 diabetes. However, the reports in this field are contradictory, and some studies have reported a significant relationship between type 2 diabetes and the ALT enzyme (20, 21).
It has been demonstrated that insufficient physical activity can lead to obesity and insulin resistance. Therefore, regular exercise can reduce the risk of cardiovascular diseases in people with diabetes (22). Patients with diabetes, particularly women, refrain from physical activities due to fear of falling and joint and bone injuries. Water training can reduce the risk of falls and fractures, decrease the pressure and stress on the joints, and facilitate joint movement in the desired range of motion (23). Babaei Bonab et al. observed that after 12 weeks of water exercise intervention, insulin resistance was improved, and there were favorable changes in liver and metabolic enzymes (24). Hitherto, most studies have investigated the effects of vitamin D supplementation or sports activities separately on liver diseases in healthy individuals. Therefore, considering the importance of physical activities and vitamin D supplementation in type 2 diabetes, further research in this field is necessary.
2. Objectives
The current study aimed to evaluate the effect of water exercise and vitamin D supplementation on liver enzyme function and the mental health of women with type 2 diabetes.
3. Methods
3.1. Participants
In our experimental research and clinical trial, 40 women (aged 40 to 60) with type 2 diabetes from the Kermanshah Diabetes Center were selected. The inclusion criteria included no smoking or history of cardiovascular and joint diseases. The exclusion criteria included: inability to continue the training program, voluntary withdrawal from the research, missing more than one training session, and not taking vitamin D supplements.
3.2. Design
One week before implementing the main protocols, we had a briefing session and obtained written consent from all subjects. The sample size was calculated by G power V. 3.1 software with 80% statistical power, 25% effect size, and 0.05 significance level.
The Ethics Committee of Kermanshah University of Medical Sciences (IR.KUMS.REC.1397.656) approved the current research, and informed consent was obtained from all subjects.
3.3. Data Collections
To identify the subjects’ diet and evaluate their mental health, the 24-hour food frequency questionnaire and Beck's Stress and Anxiety (BAI) questionnaire (21 questions were assessed on a 4-point scale (from zero to 3), alpha Cronbach 0.92), and depression (BDI-II) questionnaires (21 questions were assessed on a 4-point scale (from zero to 3), alpha Cronbach 0.87) were used before and after the exercise protocols. To measure liver enzymes, 48 hours before and 48 hours after the end of the 8-week training course, 5 ccs of blood was taken from the subjects’ antecubital vein after eight hours of fasting in a sitting resting position. The enzyme photometry method was used with commercial Pars Azmoun Company kits; Tehran, Iran.
The subjects were randomly allocated and divided (using a table of random numbers) into four groups of ten: (1) exercise in water and taking vitamin D supplements, (2) water exercise, (3) vitamin D supplementation, and (4) a control group. The whole intervention lasted for eight weeks, and the training protocol consisted of three one-hour training sessions during the week. It was carried out at an 80 - 120 cm depth in a pool with a water temperature between 26 - 28 degrees centigrade. Each training session in the water consisted of three stages, carried out in the following manner: The first stage was to adapt to the water environment and warm up for 15 minutes. The second stage was the main exercises for 30 minutes, including transferring weight from back to front, jogging, side walking, and squatting. The third stage was stretching exercises and breathing and floating movements for 15 minutes which was the cooling stage. To determine the intensity of the exercises, the Borg scale was used.
The group that took vitamin D supplements used vitamin D 50,000 IU, Zahrawi Company, Iran, weekly, and they were asked to take the supplement once a week for eight weeks. The control group did only their daily activities during the entire 8-week period.
3.4. Statistical Analysis
We use the SPSS software version 23 for this research. To evaluate the normality of the data, the Kolmogorov-Smirnov test was used. One-way analysis of variance and LSD post hoc test were used to evaluate the differences between groups. The paired t-test was used to check intragroup differences at a significance level of 0.05.
4. Results
The means and standard deviations of the descriptive characteristics of the subjects are reported in Table 1.
| Variables | Vitamin D+ Water Training | Water Training | Vitamin D | Control | P-Value |
|---|---|---|---|---|---|
| Age, y | 43.1 ± 3 | 41.5 ± 2.7 | 43.8 ± 3.6 | 42.8 ± 4 | 0.33 |
| Weight, kg | 77.7 ± 13.7 | 73.5 ± 10.8 | 72.2 ± 12.1 | 76.9 ± 6.3 | 0.02 |
| BMI, kg/m2 | 29.6 ± 65.1 | 30.1 ± 3.8 | 29.5 ± 6.4 | 30.3 ± 3.5 | 0.09 |
| BFM, kg | 32.5 ± 11.1 | 32.6 ± 6.9 | 30.1 ± 10.2 | 33. 4 ± 6.3 | 0.02 |
| SBP, mmHg | 13.5 ± 4.3 | 13.1 ± 3.7 | 12.9 ± 6.2 | 13.3 ± 5.8 | 0.21 |
| DBP, mmHg | 8.2 ± 3.8 | 7.5 ± 2.2 | 7.4 ± 3.1 | 7.9 ± 1.9 | 0.31 |
a Values are expressed as mean ± SD. P-values was obtained by ANOVA and chi-square.
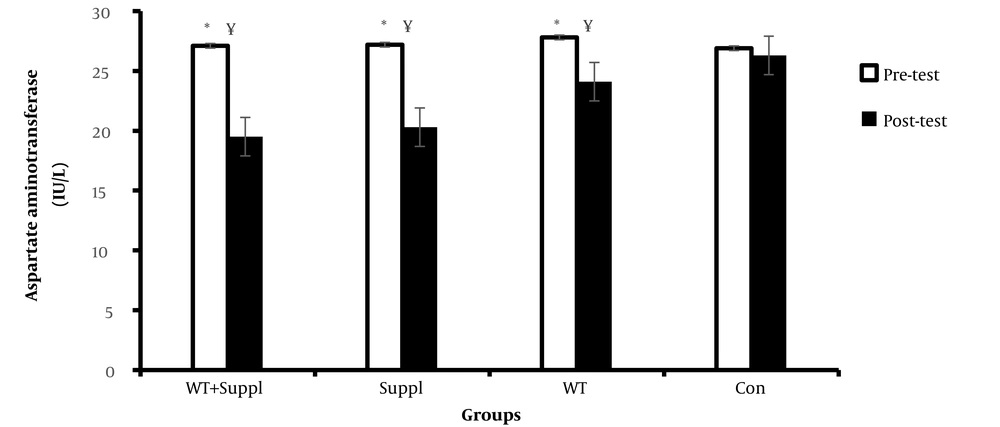
We found a significant difference in the mean concentration of aspartate aminotransferase (AST) between the four groups (F (3,36) = 12.97 and P < 0.01). The post-hoc demonstrated that the concentration of aspartate aminotransferase (AST) in the water training group + vitamin D consumption, water training group, and vitamin D consumption decreased significantly in comparison with the control group (P < 0.05). The finding of the paired t-test revealed a significant difference in the serum level of aspartate aminotransferase enzymes before and after the intervention in the water training+ vitamin D group, vitamin D group, and water training group. However, there were no significant differences in the control group in the pre-test to post-test (Figure 1).
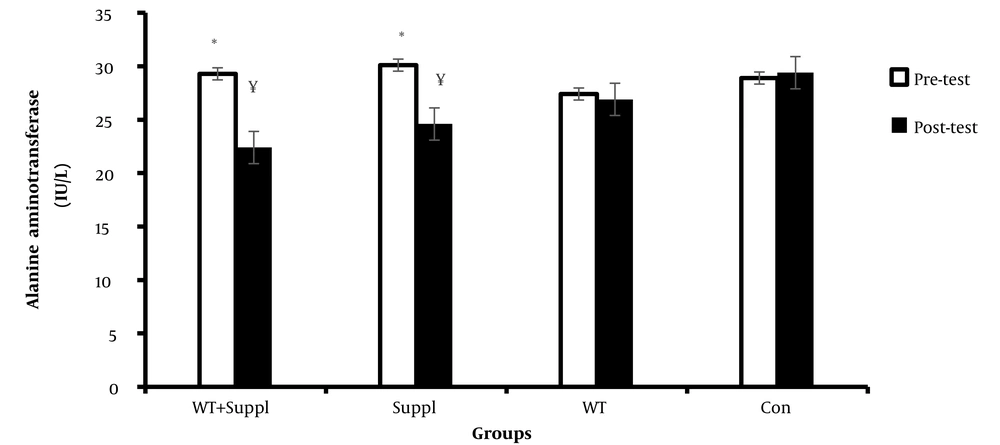
Also, we found that after the intervention, there was a significant difference in the mean of alanine aminotransferase concentration between the groups (F (3,36) = 5.71 and P < 0.001). The results of the LSD post-hoc test demonstrated that there was a significant difference in the mean concentration of alanine aminotransferase enzyme between the water training + vitamin D consumption and vitamin D consumption with the control group (P < 0.05). The paired t-test results demonstrated a significant difference in the serum levels of alanine aminotransferase enzymes before and after the interventions in the water training+ vitamin D group and vitamin D group. However, there were no significant differences between the water training group and the control group in the pre-test to post-test (Figure 2).
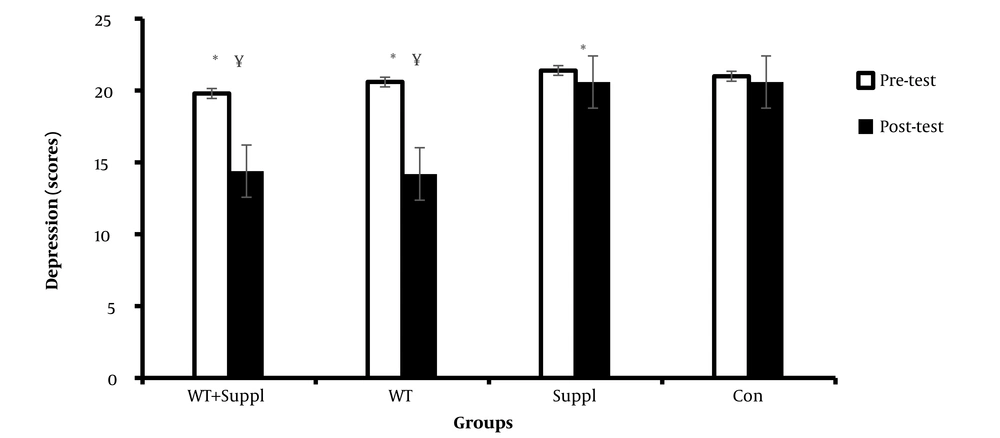
We observed a significant difference in the mean depression scores after the interventions between the groups (F (3,36) = 8.31 and P < 0.001). LSD post hoc demonstrated that there were significant differences in the means of depression scores between both the water training + vitamin D consumption group and water training group and the control group (P < 0.05). Paired t-test analysis showed significant differences in the means of depression in the three intervention groups in pre- to post-test (P < 0.05). However, there were no significant differences from pre- to post-test in the control group (P > 0.05) (Figure 3).
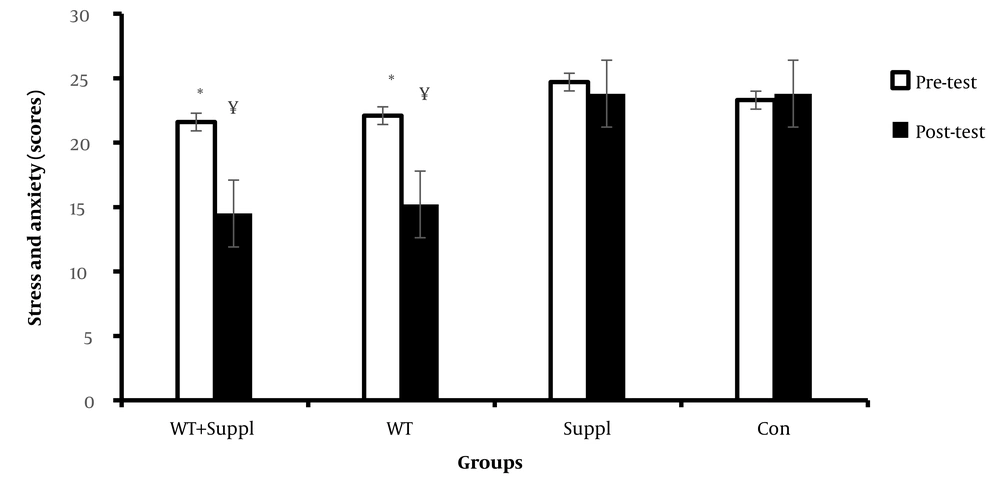
Moreover, we found a significant difference in the means of stress and anxiety scores after the interventions between the groups (F (3,36) = 6.43 and P < 0.001). LSD post-hoc demonstrated significant differences in the means of stress and anxiety scores between the water training + vitamin D consumption group and water training group compared to the control group (P < 0.05). Paired t-test analysis demonstrated significant differences in the training + vitamin D consumption and water training groups in the pre- to post-test (P < 0.05). However, there were no significant differences between the pre- to post-test in vitamin D consumption and the control group (P > 0.05) (Figure 4).
5. Discussion
The current research findings demonstrated that eight weeks of aerobic training along with vitamin D supplementation significantly reduced the level of liver enzymes compared to the control group. Similar to the current research results, in Hickman et al. research, the decrease in the serum levels of liver enzymes was associated with weight loss following 150 minutes of aerobic exercise per week (25). On the other hand, the decrease in liver enzymes caused by exercise occurs due to increased insulin sensitivity. Aerobic exercises with sufficient duration and intensity (at least 30-min and 60-80% of maximum heart rate) significantly affect insulin sensitivity and change substrate consumption in skeletal muscle (26). Similar to Eskandari et al. research, the exercise intensity in the current research was light to moderate, where the energy production system is mainly aerobic. This activity was demonstrated to be more effective on liver enzyme levels (27).
Evaluating subjects’ mental and sensory systems is widely recognized as an integral part of the behavior assessment of diabetic patients. Vitamin D is involved in various physiological and pathological processes and signaling pathways related to mental health (28). Recently, a great focus has been on the relationship between vitamin D deficiency and depression. The findings demonstrated that calcitriol plays a key role in regulating several brain neurotransmitters, including adjusting and incrementing the availability of dopamine and norepinephrine (29). Given the high prevalence of vitamin D deficiency and depression, their possible association research in this field can have significant public health implications, particularly considering that vitamin D supplement is relatively safe and cost-effective. Observational studies conducted hitherto provide some evidence for a possible relationship between vitamin D deficiency and depression (30).
In the current research, it was found that vitamin D supplementation, both alone and in combination with exercise, reduced the levels of stress, anxiety, and depression. These findings are in line with Focker et al. research, which documented the effect of vitamin D on mental health. They concluded that low vitamin D levels might lead to mental disorders and commented that restoring this level can strengthen mental health and prevent mental disorders, and consequently, it can be considered an effective therapeutic intervention (31). On the other hand, it was found that water exercise significantly reduced stress, anxiety, and depression in diabetic patients. These findings are consistent with the studies of Anglin et al. (30).
Many chronic diseases have been related to vitamin D deficiency in the past decades, including type 2 diabetes. Moreover, it has been demonstrated that the risk of diabetes increases with age and obesity. On the other hand, with lifestyle changes and reduced physical activity, the risk of diabetes will increase. All mentioned factors are common risk factors for type 2 diabetes and vitamin D deficiency, and these results confirm the correlation between vitamin D serum levels and type 2 diabetes (30, 32).
Considering the importance of depression in psychological health, which is very important in diabetes, Mohammadi et al. reported that people with diabetes are more likely to develop depression due to concerns about managing their diabetes. Therefore reducing stress and anxiety can be important in controlling type 2 diabetes (32).
5.1. Limitations
Major limitations of the current research were the small number of subjects and the inability to strictly control the subjects' nutrition during the research period.
5.2. Conclusions
Based on the finding of this research, it can be concluded that aerobic exercise, along with vitamin D supplementation, has a favorable effect on liver enzymes and depression. Therefore, exercise in water regularly can play an effective role in improving individuals’ health and quality of life. Further studies are warranted to establish whether this association is causal.