1. Background
The intensive care unit (ICU) is a special department of a hospital or healthcare facility that provides healthcare services to critically ill patients in need of life support. Admission to ICUs has significantly increased in recent years (1, 2), turning them into departments with the highest rates of mortality. It is important to consider the nutritional status of patients in the ICU since it is a key element of clinical outcomes and their ability to overcome and survive critical illnesses. From this perspective, the nutritional status of ICU patients is a significant issue that must be addressed (3). The nutrition of critically ill patients is an essential element in the holistic approach to special care. Accordingly, proper planning by considering the most effective route of administration, along with energy and nutritional need assessment, significantly enhances the prognosis and outcomes of such cases (4).
Nitrogen balance (NB) represents the rise or fall in the whole-body protein, as well as the difference between N intake and loss in the diet. Stressful situations may disrupt NB. Moreover, whole-body protein hypermetabolism and catabolism, induced by stressful events in critically ill patients, are likely to result in negative NB (5). Severe protein catabolism is typically evident in critically ill patients admitted to ICUs because protein loss is associated with higher morbidity and mortality rates (6). It seems that the higher rates of protein consumption may be associated with NB improvement and, accordingly, useful for critically ill patients, but the results are debatable (7).
Patients usually experience malnutrition in critical situations, so insufficient or improper nutritional support increases mortality rates, respiratory muscle weakness, ICU stay, sepsis (often caused by bacterial infections), multiple organ dysfunction syndrome, and even death (3). Malnutrition is common among inpatients but is sometimes overlooked because medical resources, including nutritionists or financial support, are not sufficient, or clinical professionals do not deem malnutrition critical (8). In this case, nutritional support can significantly help maintain homeostasis in ICU patients, which is why PN is recommended (9). In addition, ICU patients are highly catabolic and lose significant amounts of protein and muscle. This proteolysis may cause a 20% loss of muscle mass within a 10-day hospital stay. It is crucial to supply enough protein to critically ill patients to minimize negative NB, which is often prescribed in intestinal or injectable forms through amino acids as a part of PN (10, 11).
In keeping with such recommendations, a key treatment for ICU patients who cannot be completely and correctly fed through gastric feeding tubes is alternative PN (12), which can be divided into two types: total parenteral nutrition (TPN; exclusive), which provides all nutrients to the patients, and peripheral parenteral nutrition (PPN; partial or supplemental), in which some parts of nutrition are provided through the mouth or the intestine. These nutrients consist of amino acids, glucose, lipids, electrolytes, vitamins, and some other elements (13-15). The European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines recommend administering 1.3 g/kg/day of protein to critically ill patients, but this should be tailored to their clinical conditions (10). Besides, there is a dire need to examine the precise use of protein and calories in ICUs to boost nutrition and reduce complications and mortality (16). To the best of the authors’ knowledge, few comprehensive studies have thus far addressed the consequences of these treatment methods in ICUs; there is also a huge gap between older guidelines and current nutritional measures (9) because PN is associated with numerous complications (3).
2. Objectives
Checking patients' hemodynamic status is an essential part of treatment and nursing care and a key criterion to meet in PN (17, 18); thus, it is crucial to use a regimen based on patients' hemodynamic information (19, 20). Given the significance of monitoring the hemodynamic status (21) and maintaining its stability, especially in ICU patients, and due to the little relevant research conducted in recent years and the existence of insufficient information, it was essential to conduct this study to record new findings and documented information. Thus, the present study aimed to compare the effects of PN with the amino acid alone and amino acid-intralipid combination on the vital signs and the rate of diuresis in ICU patients.
3. Methods
3.1. Study Design
This prospective cohort study was conducted over two years, from March 2021 to March 2023. Patients who were under intravenous nutrition with amino acid (Group A) and amino acid-intralipid (Group B) for three days, as ordered by the ICU specialist, were examined.
Before starting the study, all the patients' families provided informed consent. The inclusion criteria were intubated ICU patients aged over 18 years, administration of amino acids and intralipid by their attending physicians, the absence of severe hemodynamic disorders (not taking inotropic drugs and diuretics), the absence of chronic kidney failure, and no pregnant or lactating women. Then, the ICU patients who were fed amino acid alone (Group A) or intralipid-amino acid (Group B) were compared.
Some variables, such as blood pressure (BP), heart rate (HR), respiratory rate (RR), and core body temperature (CBT), were controlled and evaluated before and after PN and recorded twice daily. The laboratory findings, i.e., sodium (Na), potassium (K), urea (U), creatinine (Cr), and blood sugar (BS) or glucose, were evaluated on the first day and at the end of the seventh day.
Before the TPN, the attending physician or anesthesiologist evaluated the hemodynamic parameters and the laboratory findings and then started the intravenous nutrition if there were no contraindications. As the main route of administration, each bag was removed from the refrigerator at least 1 - 2 h before the infusion. The TPN bags were also covered by a light protective bag to reduce vitamin degradation. All the TPN bags were subsequently discarded 24 h after the infusion, and a new TPN bag was used. The TPN flow was 60 - 80 mL/h at a constant rate. The line related to TPN was not utilized for blood and drug infusion, and this regimen was not interrupted due to the drug protocol. The patients received their maintenance therapy according to the routine schedule. The time of the amino acid and intralipid infusion was fixed (5 - 6 h) in all the patients. The patients took their medications based on the previous schedule, and there was no disruption. The rate of diuresis was measured daily before and after the infusion; then, the amount of amino acid and intralipid used in each stage was deducted from the rate of diuresis in each patient, and the remaining value was ultimately recorded.
3.2. Participants
In the two years from March 2021 to March 2023, 62 patients admitted to the ICU of Ayatollah Rouhani Hospital, Babol City, Iran , were compared and examined.
3.3. Instruments
The data were collected using two instruments. The first was the patients’ records, which contained three parts: (1) the patients' profile (age, sex, diagnosis, admission time, and hospital stay); (2) their hemodynamic parameters; and (3) laboratory findings (kidney and liver function, along with the levels of electrolytes). The second instrument was a Nutrition Assessment Form, comprising the type of nutritional regimen, the amount of absorption, excretion, and the number of times receiving the nutritional diet.
3.4. Statistical Analysis
SPSS v.26 was used for data analysis. Mean/standard deviation (SD) and frequency/percentage were utilized, respectively, to describe the quantitative and qualitative data. The normality of the data distribution was checked using the Kolmogorov-Smirnov test. One-way repeated measures analysis of variance (ANOVA), independent-samples t-test, paired-samples t-test, the Wilcoxon signed-rank test, and the Mann-Whitney U test were also applied to analyze the data. The value of P < 0.05 was considered statistically significant.
4. Results
No significant difference was observed between the two study groups with regard to age, sex, marital status, occupation, education, underlying diseases, type of surgery, and medications taken before intravenous nutrition (P < 0.05; Table 1). In the within-group comparison of the variables related to blood urea nitrogen (BUN), Cr, hematocrit (Hct), aspartate aminotransferase (AST), alanine transaminase (ALT), bilirubin (Bil), and albumin (Alb), there was a significant difference in Bil and Alb before and after the intravenous nutrition in Groups A and B (P < 0.05). There was a significant drop in Bil (from 0.85 to 0.69) and a significant elevation in Alb (from 2.69 to 3.01). A significant difference was observed in Hct, AST, and ALT in Group A (P < 0.05), which resulted in a significant increase in Hct (from 34.45 to 36.33), AST (from 49.78 to 63.21), and ALT (from 50.18 to 65.93; Table 2).
| Demographic Characteristics | Amino acid Group (A) (n = 32) | Amino Acid-Intralipid Group (B) (n = 30) | Test Results |
|---|---|---|---|
| Sex; No. (%) | X2 (1) = 0.043; P = 0.835 | ||
| Male | 20 (62.5) | 19 (63.3) | |
| Female | 12 (37.5) | 11 (36.7) | |
| Disease history; No. (%) | X2 (1) = 12.645; P = 0.068 | ||
| Yes | 20 (62.5) | 25 (83.3) | |
| No | 12 (37.5) | 5 (16.7) | |
| Diagnosis | X2 (1) = 0.022; P = 0.881 | ||
| Internal a | 9 (28.1) | 8 (26.7) | |
| Surgical b | 23 (71.9) | 22 (73.3) | |
| Age (y) (mean ± SD) | 61.66 ± 16.28 | 68.15 ± 14.99 | F = 0.25; P = 0.108 |
a Internal: Infectious, pulmonary, neurological, blood, digestive, and cardiac diseases.
b Surgical: Surgeries, including brain tumors, brain hemorrhages, orthopedics, cancers, abdominal, and gynecological.
| Laboratory Findings and Intravenous Nutrition | Amino Acid Group (A) (n = 32) | Amino Acid-Intralipid Group (B) (n = 30) | P a |
|---|---|---|---|
| BUN | |||
| Before | 32.28 ± 20.87 | 30.67 ± 18.87 | 0.725 |
| After | 33.29 ± 26.92 | 29.48 ± 22.37 | 0.394 |
| P b | 0.784 | 0.804 | |
| Cr | |||
| Before | 1.52 ± 1.12 | 1.33 ± 0.67 | 0.005 |
| After | 1.33 ± 0.46 | 1.16 ± 0.62 | 0.043 |
| P b | 0.362 | 0.774 | |
| Hct | |||
| Before | 34.45 ± 4.91 | 33.69 ± 13.67 | 0.024 |
| After | 36.33 ± 4.45 | 32.03 ± 10.51 | < 0.001 |
| P b | 0.029 | 0.804 | |
| AST | |||
| Before | 49.78 ± 26.44 | 66.20 ± 45.94 | 0.326 |
| After | 63.21 ± 22.39 | 62.86 ± 45.91 | 0.198 |
| P b | 0.002 | 0.713 | |
| ALT | |||
| Before | 50.18 ± 23.13 | 61.13 ± 67.96 | 0.360 |
| After | 65.93 ± 21.23 | 53.76 ± 47.03 | 0.012 |
| P b | < 0.001 | 0.416 | |
| Bil | |||
| Before | 0.50 ± 0.20 | 0.85 ± 0.36 | 0.001 |
| After | 0.53 ± 0.20 | 0.69 ± 0.24 | 0.001 |
| P b | 0.239 | 0.045 | |
| Alb | |||
| Before | 4.83 ± 5.58 | 2.69 ± 0.54 | 0.001 |
| After | 4.00 ± 0.84 | 3.01 ± 0.60 | 0.001 |
| P b | 0.300 | 0.001 |
a Significance level, the Mann-Whitney U test.
b Significance level, the Wilcoxon signed-rank test.
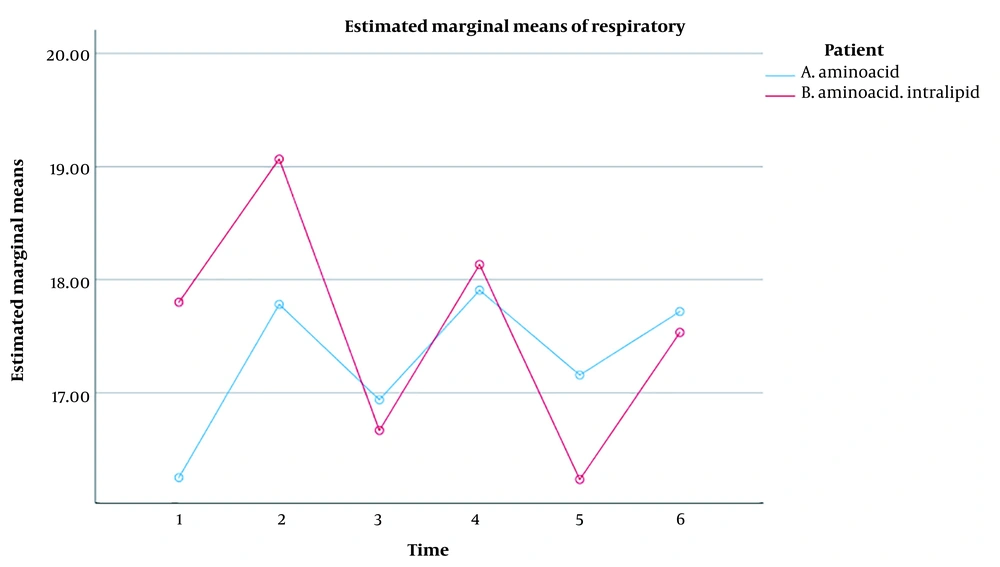
The repeated measures ANOVA revealed the significant effect of the measurement time on the RR of the ICU patients (P < 0.001). Regardless of the grouping, a significant difference was found between the mean RR in the patients during the six-time measurement. In this respect, the repeated measures ANOVA showed a significant difference in the six-time measurement of RR in Groups A (P = 0.017) and B (P = 0.013). In addition, the time x group interaction effect on RR in such patients was not significant (P = 0.062). Therefore, there was no difference between the mean RR in the two groups at different times. Besides, the group's effect on RR in the ICU patients was not significant (P = 0.769). Consequently, no significant difference was detected between the two groups while not considering the measurement times (Table 3).
| Time | RR (Mean ± SD) | Test Value a | Repeated Measures ANOVA | |||
|---|---|---|---|---|---|---|
| Amino Acid Group (A) | Amin Acid-Intralipid Group (B) | Within-Group | Between-Group | |||
| Time Effect | Time × Group Effect | Group Effect | ||||
| Stage 1 | 16.26 ± 3.79 | 17.80 ± 5.49 | F = 3.638; P = 0.199 | F = 4.969; P < 0.001 | F = 2.307; P = 0.062 | F = 0.087; P = 0.769 |
| Stage 2 | 17.78 ± 3.59 | 19.06 ± 4.74 | F = 3.297; P = 0.233 | |||
| Stage 3 | 16.93 ± 3.86 | 16.66 ± 5.10 | F = 2.129; P = 0.814 | |||
| Stage 4 | 17.90 ± 3.89 | 18.13 ± 4.65 | F = 2.069; P = 0.835 | |||
| Stage 5 | 17.15 ± 3.38 | 16.23 ± 4.58 | F = 3.544; P = 0.368 | |||
| Stage 6 | 17.71 ± 4.01 | 17.53 ± 4.93 | F = 1.023; P = 0.871 | |||
| Repeated measures ANOVA | F = 3.288; P = 0.017 | F = 3.700; P = 0.013 | - | |||
a Significance level, independent-samples t-test.
Moreover, RR in Group A significantly increased from the onset to the end of the intravenous nutrition (P < 0.05), but this value significantly decreased from the beginning to the end of the intravenous nutrition in Group B (P < 0.05). It was thus concluded that the effect of both infusions on RR was the same over time (Figure 1).
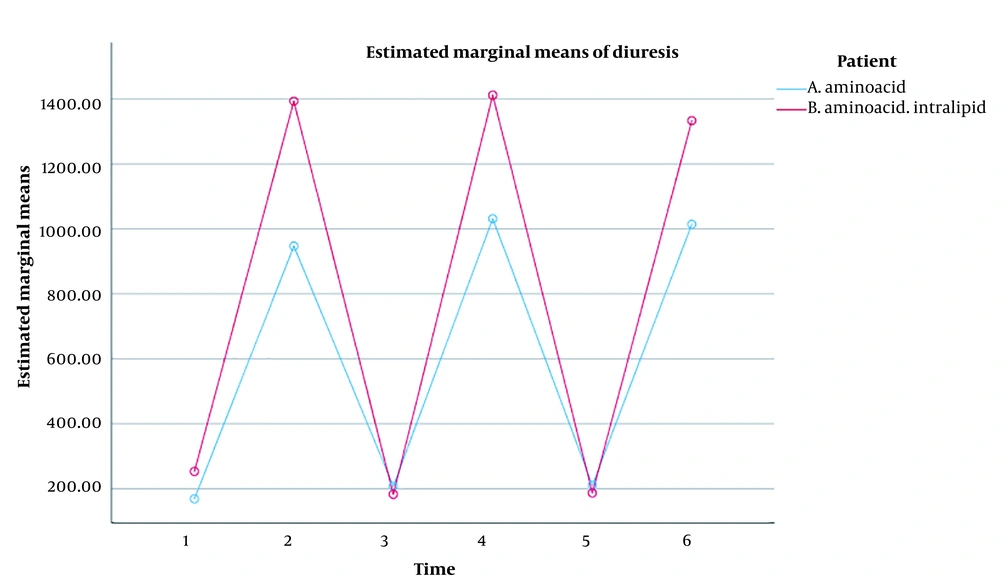
The repeated measures ANOVA results illustrated that the effect of measurement time on the rate of diuresis in ICU patients was significant (P < 0.001). Irrespective of the grouping, there was a significant difference between the mean rate of diuresis in the ICU patients, which indicated a significant difference in the six-time measurement of the rate of diuresis in Groups A (P < 0.001) and B (P < 0.001). Correspondingly, the results confirmed that the time x group interaction effect on the rate of diuresis in the ICU patients was significant (P < 0.001). Therefore, a significant difference was established between the mean rates of diuresis in both groups at different times. Besides, the group's effect on the rate of diuresis in ICU patients was significant (P 0.001). As a result, a significant difference was observed between the two groups, regardless of the measurement times (Table 4).
| Time | Rate of Diuresis | P a | Repeated Measures ANOVA | |||
|---|---|---|---|---|---|---|
| Within-Group | Between-Group | |||||
| Amino Acid Group (A) | Amin Acid-Intralipid Group (B) | Time Effect | Time × Group Effect | Group Effect | ||
| Stage 1 | 168.75 ± 223.87 | 253.33 ± 284.03 | F = -1.064; P = 0.287 | F = 194.604; P 0.001 | F = 7.379; P < 0.001 | F = 14.275; P < 0.001 |
| Stage 2 | 946.87 ± 319.51 | 1393.33 ± 487.73 | F = -3.456; P = 0.001 | |||
| Stage 3 | 207.81 ± 264.79 | 182.66 ± 264.79 | F = -0.431; P = 0.666 | |||
| Stage 4 | 1031.25 ± 306.58 | 1411.66 ± 537.18 | F = -3.107; P = 0.002 | |||
| Stage 5 | 210.93 ± 216.17 | 186.66 ± 314.29 | F = -1.012; P = 0.311 | |||
| Stage 6 | 1014.06 ± 321.34 | 1333.33 ± 465.04 | F = -2.550; P = 0.011 | |||
| Repeated measures ANOVA | F = 96.646; 0.001 > P | F = 100.002; 0.001 > P | - | |||
a Significance level, the Mann-Whitney U test.
Overall, the rate of diuresis in both groups had an upward and downward trend. No significant difference was detected between the two groups in the first, third, and fifth stages, but there was a significant difference in the second, fourth, and sixth stages, i.e., after the infusions, and the rate of diuresis was significantly higher in Group B (Figure 2).
5. Discussion
The present study investigated the effects of PN with the amino acid alone and amino acid-intralipid combination on the vital signs and the rate of diuresis of ICU patients. As for the first objective, the use of the amino acid alone increased the RR of these patients. There was no significant difference in BP, HR, and temperature after PN, but an upward trend was observed in both groups over time. Consistent with these findings in terms of BP, Lin et al. provided evidence for the causal effects of higher levels of amino acids on higher BP and the risk of developing high BP (P < 0.006) (22). In line with the results regarding RR, Takala et al., investigating the effects of PN with standard and branched amino acid on the metabolism and ventilation of ICU patients, showed that the use of both PNs increased RR and oxygen consumption but decreased carbon dioxide (CO2) level (23). Unlike the findings about the temperature in other studies, this study concluded that the ICU patients had less postoperative temperature drop and shivering after receiving the amino acid (24, 25). The difference in the findings could be attributed to the temperature variations in the study settings (operating rooms in the previous studies versus the ICU in the current study).
The second objective was to examine the effect of PN with amino acid-intralipid on the rate of diuresis in ICU patients. In this vein, the rate of diuresis in the patients receiving amino acid-intralipid was higher compared with those infused with amino acid alone, which reduced the BUN level much more. In contrast to the findings of the present study, Singer et al. concluded that the daily use of 150 vs. 75 mg of amino acids in patients with kidney diseases in the ICU augmented the rate of diuresis (26). In the present study, this was more evident in Group B, receiving PN with amino acid-intralipid. The discrepancy in these results is probably due to the dose and type of PN in both groups because Groups A and B faced an increase in the rate of diuresis in the present study.
The findings also revealed a significant difference in the level of BUN before and after intravenous nutrition in Group A (P < 0.001), from 33.45 ± 20.87 mg/dL to 38.79 ± 26.92 mg/dL. Contrary to these findings, Suzuki et al. found that the group receiving more than 1.2 g/kg of protein per day had a higher level of BUN at the end of the week than the group infused with less than this amount (11). Moreover, Singer et al. concluded that the gradual changes in BUN from the first to the fourth days in the group receiving 75 mg of amino acid were amplified, compared with the group receiving 150 mg (P < 0.01). In addition, the trend of Cr changes was ascending but not significant (26). The contradictory results in terms of the increasing or decreasing changes could be related to the levels of BUN and Cr in the specific elements of each treatment because fluids with multiple compounds can even change the rate of diuresis. The early consumption of high-protein nutrition could possibly improve the prognosis of ICU patients, but the high intake of protein could elevate the levels of BUN (11) because the consumption of protein and PN with amino acids can dilate renal vessels with subsequent growth in the renal plasma flow (RPF) and glomerular filtration rate (GFR) (27, 28).
As for the liver enzymes, the findings of this study revealed that the administration of PN with amino acid-intralipid decreased the ALT, AST, and Bil, which was in line with the report of Liu et al. The combination itself could further reduce ALT and AST (29). In addition, Navaratnarajah et al. found that the children using SMOFlipid-intralipid had lower BL levels at the end of the study (30); however, Hsu et al. established that amino acid administration had no effect on liver tests (31). The reason for the difference in results could be attributed to the types of diseases affecting the patients, as some studies were conducted on cases with liver diseases, preterm infants, or in animal laboratory environments.
5.1. Limitations
The present study faced several limitations. First, the patients who expired within one week after EN were excluded, which might have caused selection bias. In addition, no information on oral intake was collected, which might be a source of bias. Due to the limited sample size, all the ICU patients who met the inclusion criteria in both groups were recruited, but it would have been more appropriate to compare patients with the same diagnosis.
5.2. Conclusions
PN with amino acid alone or in combination with intralipid was effective for ICU patients. Although PN with amino acid-intralipid increased the rate of diuresis and then decreased RBC in the patients, the cases receiving amino acid alone experienced a rising trend in the rate of diuresis, SBP, and RR. Accordingly, it seems more economical to prescribe PN with amino acid alone, except for patients with kidney diseases, whereas the combined amino acid-intralipid regimen is much more useful for augmenting the rate of diuresis and RBC. The patients who took amino acid showed a growth in the laboratory findings, i.e., ALT, AST, and Bil, as well an elevation in RR and SBP, which improved their hemodynamic status. As a result, it seems more economical to prescribe PN with intralipid or amino acid-intralipid in a targeted manner based on the patients' health conditions (particularly liver and kidney diseases) because the use of each one alone may benefit or harm them.