1. Background
Sleep disorder is frequent during pregnancy, which may occur from the 12th week of pregnancy to the postpartum period, often in the form of difficulty in falling asleep, frequent waking ups, and insomnia (1). Sex hormones and gonadotropin can affect sleep quality and contribute to sleep disorders, such as insomnia. Hence, hormonal and physiological changes caused by pregnancy can affect a woman’s ability to sleep (2). Changes in sleep during pregnancy are caused by various changes in the body and mind. These changes include differences in body structure, how the body works, hormones, and thoughts and emotions. Gaining more weight (up to 20% more than pre-gestational weight) and having a larger uterus are the biggest anatomical changes. The bigger uterus can push up on the diaphragm and make it harder to breathe (3). Sleep difficulties can be encountered when experiencing trouble with bodily movements during sleep, leading to disrupted sleep and difficulty falling asleep. The main physical factors include changes in the heart rate, blood pressure, and breathing rate, with a higher difference in oxygen levels between the alveoli and the arteries. During pregnancy, there is an increase in sympathetic activity, which is partly caused by hormonal changes. This also leads to an increase in cardiac load and ejection fraction. All of these changes make the expecting mother feel tired and worn out (4).
According to the National Sleep Foundation of America, over 79% of pregnant women have reported changes in their sleep patterns or sleep problems that they did not experience before they became pregnant. Over the past decade, scientists have found a complicated connection between being pregnant and sleep disturbances, indicating possible adverse pregnancy-related outcomes, including preeclampsia, cesarean delivery, gestational diabetes mellitus (GDM), preterm birth, and lower birthweight (5-7). Sharma and Franco reported that 79% of pregnant women suffer from a sleep disorder (8), a rate of 61.5% reported for Iranian women by Akbari et al. (9).
Sleep disorders during pregnancy have adverse effects on the health of the mother and fetus (10). Several studies reported an association between sleep disorder during the third trimester with depression, reduced pain tolerance, suppression of emotions, prolonged labor, increased probability of cesarean section, childbirth bolus, low birth weight, preterm delivery, and hypertension. However, the evidence is still inconclusive (11-15). For instance, Fung et al. reported an association between sleep disorder and low birth weight (13); however, Franklin et al. found no association (15).
The stress experienced by pregnant individuals can be attributed to several factors, including financial constraints, demanding occupations, limited educational opportunities, inadequate prenatal care, substance dependency, and relationship difficulties (16). Studies indicate a link between inadequate sleep and heightened stress levels, both of which can have detrimental effects on the immune system and hormone regulation in the body. It can also increase the risk of getting sick (17). Sleep is probably an outcome and an indicator of higher levels of stress during pregnancy, which could lead to a series of bad outcomes for the birth, the mother, and the child (18).
Due to the high morbidity and possible adverse perinatal outcomes of maternal sleep disturbances, this issue has become a noticeable research interest in recent years, resulting in dozens of observational studies and several meta-analyses. It is crucial to highlight that the outcomes are ambiguous, with certain results conflicting with others. (19).
2. Objectives
Considering the importance of the physical and mental health of pregnant women, which is a significant task for healthcare providers, identifying factors that contribute to this problem is vital. In this line, the current study aimed to investigate the association of sleep disorders with pregnancy outcomes during the third trimester.
3. Methods
3.1. Study Desighn
In this descriptive-analytical study, 100 pregnant women referring to the Mousavi Educational Hospital, affiliated with the Zanjan University of Medical Sciences, from April to September 2021 were recruited by convenience sampling.
3.2. Participants
The inclusion criteria were the third trimester of pregnancy, the age of the mother between 15 - 45, no history of sleep disorder, no history of infertility, not using in vitro fertilization, no record of psychological problems (i.e., depression and anxiety), and no comorbidity, including hypertension, diabetes before pregnancy, cardiovascular diseases, kidney diseases, and autoimmune diseases. The exclusion criteria were not filling out the questionnaire.
3.3. Data Collection
The study tools used in this study include the demographic information and obstetric history, the Pittsburgh Sleep Quality Index, and the checklist of pregnancy outcomes.
The demographic information and obstetric history included age, weight, height, gestational age, gravid, para, and abortion history. To assess sleep quality, we used the 19-item self-reported PSQI questionnaire that measures the quality and patterns of sleep over a one-month duration. The PSQI has been extensively utilized in research examining the health of large cohorts from the general populace. It contains 19 things and 7 parts about sleeping, including how well you sleep, how long you sleep, how quickly you fall asleep, things that disturb your sleep, how well you sleep, if you take medicine to help you sleep, and how tired you feel during the day. Scores ranging from 0 to 3 are assigned to each part of the rephrase. A global score for overall sleep quality can be calculated by the sum of all these components, yielding scores ranging from 0 to 21. PSQI global scores of greater than 5 are generally used to indicate poor sleep, and we adopted this same threshold in our study. The Korean version of the PSQI has shown high sensitivity and specificity and has been validated previously (20). The use of a cut-off point of 5in the Korean population has also been validated in Choi et al.’s study (21). In the study of Simoncini et al., the Pearson correlation coefficient in the test-retest method for the Pittsburgh Sleep Quality Questionnaire was 16%, and Cronbach’s alpha was 0.77% (22). Cronbach’s alpha was calculated at 86% in this study. The outcome checklist included preterm labor, intrauterine growth delay (IUGR), LBW, hypertension, preeclampsia, and diabetes.
3.4. Data Analysis
Data were analyzed using descriptive statistics and the chi-square test by SPSS version 24. The significance level was considered at P ≤0.05.
3.5. Ethical Consideration
The research purpose and methodology were subjected to scrutiny by the Ethics Committee of the Zanjan University of Medical Sciences (code: IR.ZUMS.REC.1400.234). Before entering the study, written informed consent was obtained from all participants after a comprehensive presentation of the study protocol. In addition, the assurance was given to them that their information would be guarded confidentially. Then, they were asked to fill out the questionnaires. Further information was obtained through investigating patients’ medical records.
4. Results
A total of 100 pregnant women in the third trimester were recruited. Approximately half of the participants (46 (46%)) were at the age of 36 years and older, 62 (62%) participants were nulligravid, and 65 (65%) participants were nulliparous. The gestational age of 44 (44%) participants was between 32 - 36 weeks (Table 1).
| Characteristics | No. (%), N = 100 |
|---|---|
| Age (y) | |
| Less than 25 | 18 (18) |
| 25 to 35 | 36 (36) |
| 36 and older | 46 (46) |
| Height | |
| Less than 160 | 24 (24) |
| 160 to 165 cm | 62 (62) |
| 166 and taller | 14 (14) |
| Weight | |
| Less than 60 kg | 16 (16) |
| 60 to 70 kg | 41 (41) |
| 70 to 80 kg | 16 (16) |
| 80 to 90 kg | 18 (18) |
| More than 90 kg | 9 (9) |
| Gravid | |
| 1 | 62 (62) |
| 2 | 29 (29) |
| 3 | 6 (6) |
| 4 ≤ | 3 (3) |
| Para | |
| 0 | 65 (65) |
| 1 | 32 (32) |
| 2 | 3 (3) |
| Abortions | |
| 0 | 79 (79) |
| 1 | 19 (19) |
| 2 | 2 (2) |
| Gestational age (weeks) | |
| Less than 32 | 34 (34) |
| 32 to 36 | 44 (44) |
| More than 36 | 22 (22) |
According to the findings, the sleep quality of 45 (45%) participants was relatively good for 36 (36%) participants and fairly bad to poor for 19 (19%) participants. Moreover, 81 (81%) participants reported more than 6 hours of sleep duration, 11 (11%) reported 5 to 6 hours, and 4 participants reported less than five hours of sleep duration. Also, 84 (84%) participants reported a falling asleep duration of fewer than 30 minutes. However, this time was more than 30 minutes for 16 (16%) participants. In addition, 85 (85%) participants reported a sleep efficiency of more than 85%.
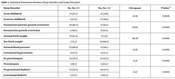
In this study, the mean (SD) of sleep quality was 5.11 (3.07), which indicates poor sleep quality. More than half of the participants (55 (57%)) had a mild sleep disorder, and 67 (67%) participants believed in no association between sleep duration and daily activities; meanwhile, 42 (42%) participants did not use sleeping pills (Table 2). There was a significant association between preterm childbirth, intrauterine growth restriction, low birth weight, gestational hypertension, preeclampsia, gestational diabetes, and sleep disorders (P-value < 0.05) (Table 3).
| Pittsburgh Sleep Quality Index Components | Score | No. (%) |
|---|---|---|
| Subjective sleep quality | ||
| Very good | 0 | 36 (36) |
| Fairly good | 1 | 45 (45) |
| Fairly bad | 2 | 12 (12) |
| Vera bad | 3 | 7 (7) |
| Sleep duration | ||
| More than 7 hours | 0 | 44 (44) |
| 6 to 7 hours | 1 | 41 (41) |
| 5 to 6 hours | 2 | 11 (11) |
| Less than 5 hours | 3 | 4 (4) |
| Sleep efficacy | ||
| More than 85% | 0 | 58 (58) |
| 57% to 84% | 1 | 27 (27) |
| 65% to 74% | 2 | 14 (14) |
| Less than 65% | 3 | 1 (1) |
| Sleep latency | ||
| ≤ 15 minutes | 0 | 39 (39) |
| 16 to 30 minutes | 1 | 45 (45) |
| 31 to 60 minutes | 2 | 14 (14) |
| More than 60 minutes | 3 | 2 (2) |
| Daytime dysfunction | ||
| 0 | 67 (67) | |
| 1 | 22 (22) | |
| 2 | 7 (7) | |
| 3 | 4 (4) | |
| Sleep disturbance | ||
| 0 | 30 (30) | |
| 1 | 57 (57) | |
| 2 | 13 (13) | |
| 3 | 0 (0) | |
| Use of sleep medication | ||
| Never | 0 | 42 (42) |
| Less than once a week | 1 | 39 (39) |
| Once or twice a week | 2 | 14 (14) |
| Three or more times a week | 3 | 5 (5) |
| Sleep Disorder | No, No. (%) | Yes, No. (%) | Chi-square | P-Value a |
|---|---|---|---|---|
| Term childbirth | 72 (83.7) | 14 (14/3) | 24.91 | 0.0001 |
| Preterm childbirth | 3 (21.4) | 11 (78.6) | ||
| Normal intrauterine growth restriction | 70 (80.5) | 17 (19.5) | 10.64 | 0.0001 |
| Intrauterine growth restriction | 5 (38.5) | 8 (61.5) | ||
| Normal birth weight | 73 (92.4) | 6 (7.6) | 60.78 | 0.0001 |
| Low birth weight | 2 (9.5) | 19 (90.5) | ||
| Normal blood pressure | 72 (80.9) | 17 (19.1) | 15.01 | 0.0001 |
| Gestational hypertension | 3 (27.3) | 8 (72.7) | ||
| No preeclampsia | 74 (78.7) | 20 (21.3) | 12.21 | 0.0001 |
| Preeclampsia | 1 (16.7) | 5 (83.3) | ||
| No gestational diabetes | 72 (78.3) | 20 (21.7) | 6.52 | 0.0001 |
| Gestational diabetes | 3 (37.5) | 5 (62.5) |
a Chi-square test
5. Discussion
This study demonstrated a significant association between study outcomes (preterm delivery, low birth weight, intrauterine growth delay (IUGR), gestational diabetes, gestational hypertension, and preeclampsia) and sleep disorders (P-value = 0.001).
Sleep disorder was more common in women with preterm delivery. In a study with 2,265 participants, Maxman found that sleep disorder was two times higher in women with preterm delivery than their counterparts with standard delivery (23). Similar findings are reported by Sherma and Franco in India and Najar et al. in Iran (8, 24). On the contrary, no significant association has been reported between sleep disorders in two groups of preterm and term delivery in the Naud et al. study (25). The observed difference can be attributed to the methodological issues (i.e., the current study followed a cohort design) and study population.
Sleep disorder was more common in women with normal-weight children than those with low birth weight. Similar findings are reported by Dabaghi et al. (26) and Parsai et al. (27). However, in Najar et al.’s and Hajipour et al.’s studies, there was no significant association between sleep disorder and preterm delivery, such association is not found for low birth weight (24, 28). This difference can be attributed to the use of a different questionnaire by Najar et al. In addition, they only considered the last month of pregnancy, while this study contained the third trimester.
Based on the findings, sleep disorder was more common in women with IUGR. Berbets, in a study on 80 women with IUGR, reported a significant association with a sleep disorder during pregnancy and IUGR (29). Kneitel et al. reported a significant association between sleep apnea during pregnancy in women suffering from IUGR. Healthy fetuses generally well transient hypoxemia of the mother, while it causes damage in other fetuses, mainly caused by sleep apnea (30). Following a meta-analysis design, Ghante et al. emphasized a significant association between IUGR and sleep disorders (31). In this study, sleep disorder was more prevalent in women with gestational diabetes, which is in line with Gante's study.
Ghanei Gheshlagh et al. and Li et al. reported a significant association between sleep apnea and gestational diabetes in pregnant women (32, 33). Sharma et al. also reported an association between gestational diabetes and sleep disorders (34). However, Hajipour et al. disclosed no association between sleep disorders and gestational diabetes (28). The difference can be attributed to the study design. Hajipour et al. followed a multi-center design, recruited 3,675 participants from 11 cities, and used a researcher-developed questionnaire with five items. The findings of the present study showed that sleep disorder was more prevalent in women with gestational hypertension and preeclampsia, consistent with several studies (32-35). In a study on the association between gestational hypertension and preeclampsia, Kneitel et al. reported no statistically significant difference (30).
5.1. Limitations
Some limitations and challenges must be considered before generalizing the findings, including a small sample size (n = 100); however, larger sample sizes are needed to generalize the findings. The COVID-19 pandemic hindered us from psychologically evaluating participants as a potential confounding factor. Partial filling of sample sizes resulted in attrition of the sample size. Another significant limitation is the use of a self-reported questionnaire.
5.2. Conclusions
As a frequent disorder during pregnancy, sleep disorder causes negative impacts. Hence, more attention should be paid to prenatal consultations and increasing the awareness of pregnant women. It is suggested that this issue has to be studied in the form of qualitative studies, and related factors of sleep quality and pregnancy outcomes should be controlled.