1. Background
According to the report of the International Diabetes Federation, the prevalence of diabetes in the adult population (20 - 79 years) in 2019 was 9.3% (463 million patients), and it is predicted that this rate will reach 10.2% (578 million patients) in 2030 and 10.9% (700 million patients) in 2045 (1). Diabetes mellitus (DM) is caused by defects in insulin secretion, insulin receptors, or a combination of them. This chronic and long-term disease is generally characterized by high blood sugar (hyperglycemia), insulin resistance, and decreased insulin secretion, making it the third most common non-communicable disease in the world after cardiovascular diseases and cancer (1, 2).
Type 2 diabetes is related to other chronic diseases such as obesity, high blood pressure, high cholesterol, high triglycerides, hyperuricemia, insulin resistance, and more, and is considered a risk factor for these diseases (3). The most important and common cause of end-stage renal failure is diabetic nephropathy, which manifests as glomerular hyperfiltration, microalbuminuria, overt albuminuria, and a gradual decrease in glomerular filtration (4). About half of diabetic patients will develop symptoms of kidney damage (diabetic nephropathy) during their lifetime (5). Following diabetic nephropathy, the serum levels of urea, uric acid, and creatinine increase in patients (6).
Uric acid is a weak acid that is naturally excreted by the kidneys, with two-thirds of urate, the ionized form of uric acid, excreted by the kidneys and the rest through bile, and secretions in the stomach and intestines (4). Various studies have pointed out the relationship between uric acid and metabolic syndrome, type 2 diabetes, and insulin resistance (3), and it is known as a risk factor for diabetes and hyperglycemia in the general population (1, 7). Hyperuricemia is associated with preglomerular vascular damage, glomerular hypertension, and decreased renal tissue perfusion, leading to interstitial fibrosis (2-4).
Hyperuricemia is associated with peripheral arterial diseases, hypertension, hypertriglyceridemia, high HbA1C, increased albuminuria, low glomerular filtration, and rapid progression of nephropathy, which is considered a risk factor for renal dysfunction (8, 9).
Regarding the correlation of serum uric acid levels with diabetes and chronic hyperglycemia, conflicting results have been reported. In the study by Haque et al., considering that the serum level of uric acid in healthy people was higher than that of pre-diabetic and diabetic people, they concluded that the serum level of uric acid is not a good predictor of diabetes in people. Considering the contradictions in the results of past studies and the lack of sufficient information regarding the relationship between hyperuricemia and hypertension, this study was conducted to investigate the relationship between hyperuricemia and hypertension in diabetic patients undergoing hemodialysis treatment (10). In the study by Rao and Sahayo, serum uric acid levels were higher in pre-diabetes than in healthy subjects and lower in diabetes than in pre-diabetes (11).
2. Objectives
Considering the contradictions in the results of past studies and the lack of sufficient information regarding the relationship between hyperuricemia and hypertension, this study was conducted to investigate the relationship between hyperuricemia and hypertension in diabetic patients undergoing hemodialysis treatment.
3. Methods
3.1. Study Design
In this descriptive-analytical cross-sectional study, the study population comprised dialysis patients referred to the Comprehensive Treatment Center for Special Diseases in Birjand in 2022. A total of 70 eligible individuals were selected using the available sampling method.
3.2. Participants
Patients over 18 years old diagnosed with diabetic nephropathy and without a history of kidney transplantation, who provided consent to participate in the study, were included. Patients with a history of cardiovascular disease, prior use of diuretics, previous use of uric acid-lowering medications, gout, or alcohol consumption were excluded from the study.
Considering that different studies reported varying correlation coefficients between blood pressure and uric acid, ranging from 0.3 to 0.5, and to calculate the maximum sample size with a power of 80% and confidence limits of 95%, assuming a correlation coefficient of 0.35, the sample size was determined to be 61 individuals. Accounting for potential incomplete cases, the final sample size was adjusted to 70 individuals.
3.3. Scales
Serum uric acid levels > 6 mg/dL are considered hyperuricemia, while blood pressure exceeding 140/80 mmHg before initiating dialysis is classified as hypertension. Patients with a confirmed diagnosis of diabetes who were receiving insulin therapy before dialysis were considered diabetic.
Following an explanation of the study's implementation method, informed consent was obtained from the patients to participate in the study. Subsequently, demographic information was collected from the patients and recorded in the appropriate checklist. After allowing the patients to sit and rest for 15 minutes, blood pressure was measured by the researcher (a medical student) using a cuff and a medical stethoscope (Alp k2). Blood samples were then collected from the patients in a fasting state (10 - 12 hours), and their serum was isolated. The serum uric acid level was measured using the Bionic company kit manufactured in Iran and the Prestige 24 machine made in Japan. Finally, all necessary information was documented in the checklist for each patient.
3.4. Data Collection
The required information was obtained from the patients' files using a checklist. In cases where the files were incomplete, the missing data were gathered through interviews with the patients. The checklist comprised two sections: Demographic information and studied indicators. Demographic details encompassed age, sex, duration of hemodialysis, duration of diabetes, and history of underlying diseases. The studied indicators included systolic blood pressure, diastolic blood pressure, blood uric acid levels, and blood sugar levels.
3.5. Data Analysis
Data analysis was conducted using SPSS 25 software. Descriptive statistics, including frequency percentage, mean, and standard deviation (SD), were utilized for data description. The normality of data distribution was assessed using the Kolmogorov-Smirnov test. Data analysis was performed employing the chi-square test, Fisher's exact test, Kruskal-Wallis test, and Mann-Whitney U test. A significance level of 0.05 was applied for all analyses.
3.6. Ethical Consideration
This study was approved by the ethics committee of Birjand University of Medical Sciences with the code IR.BUMS.REC.1401.020. Additionally, participants completed the informed consent form before the study commenced, and they were assured of the confidentiality of their information and that it would not be disclosed individually.
4. Results
Most of the patients were male (44, 62%) and in the age group of 50 - 70 years (39, 54.9%). The duration of dialysis in most patients was more than 3 years (39, 54.9%), and most of them were under treatment with insulin (39, 54.9%). Sixty-two (85.9%) patients had hypertension. The average blood pressure in the patients was 114.9 ± 55.8, with the average systolic and diastolic blood pressure being 130.7 ± 18.7 and 79.1 ± 10.1, respectively. The average uric acid level was 5.9 ± 1.12, and hyperuricemia was observed in 11 (15.5%) of the patients. More details are provided in Table 1.
| Descriptive Variables | Values |
|---|---|
| Age (y) | |
| < 50 | 8 (11.3) |
| 50 - 70 | 39 (54.9) |
| ≥ 70 | 24 (33.8) |
| Sex | |
| Female | 27 (38) |
| Male | 44 (62) |
| Dialysis duration (y) | |
| < 3 | 32 (45.1) |
| ≤ 3 | 39 (54.9) |
| Hypertension (high blood pressure) | |
| No | 10 (14.1) |
| Yes | 61 (85.9) |
| Treated with insulin | |
| No | 32 (45.1) |
| Yes | 39 (54.9) |
| Hyperuricemia | |
| No | 60 (84.5) |
| Yes | 11 (15.5) |
| Quantitative variable | |
| Uric acid (mg/dL) | 5.99 ± 1.12 |
| Blood sugar (mg/dL) | 114. 9 ± 55.8 |
| Systolic blood pressure (mmHg) | 130.7 ± 18.7 |
| Diasystolic blood pressure (mmHg) | 79.1 ± 10.12 |
a Values are expressed as No. (%) or mean ± SD.
In general, the rate of hyperuricemia was higher in patients with high blood pressure (16.4% vs. 10%). However, according to Fisher's exact test, there was no significant relationship between hyperuricemia and high blood pressure (P = 0.605) (Table 2).
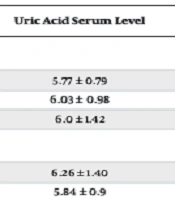
The average level of uric acid was higher in the age group of < 50 years (6.2 ± 1.1 mg/dL) and ≥ 70 years (6.1 ± 1.42 mg/dL), in female patients (6.25 ± 1.39 mg/dL), and in patients with < 3 years of dialysis (6.1 ± 1.28 mg/dL). However, based on the results of the Kruskal-Wallis test, there was no significant relationship between age and serum uric acid level (P = 0.173). Furthermore, according to the results of the chi-square test, no significant difference between gender and duration of dialysis with serum uric acid level was observed (P > 0.05) (Table 3).
| Variables | Uric Acid Serum Level | Test Statistics | P-Value |
|---|---|---|---|
| Age (y) | 0.391 | 0.822 b | |
| < 50 | 5.77 ± 0.79 | ||
| 50 - 70 | 6.03 ± 0.98 | ||
| ≥ 70 | 6.0 ± 1.42 | ||
| Sex | 479 | 0.173 c | |
| Female | 6.26 ± 1.40 | ||
| Male | 5.84 ± 0.9 | ||
| Dialysis duration (y) | 590 | 0.694 b | |
| < 3 | 6.07 ± 1.29 | ||
| ≤ 3 | 5.94 ± 0.98 | ||
| Systolic blood pressure (mmHg) | 394 | 0.948 b | |
| < 140 | 6.02 ± 1.2 | ||
| ≤ 140 | 5.9 ± 0.91 | ||
| Diastolic blood pressure (mmHg) | 111.5 | 0.237 c | |
| < 90 | 6.03 ± 1.13 | ||
| ≤ 90 | 5.5 ± 0.89 |
a Values are expressed as mean ± SD.
b Kruskal-Wallis test.
c Mann-withney U test.
The average uric acid level was higher in patients with systolic blood pressure less than 140 mm Hg (6.02 ± 1.2) and in patients with diastolic blood pressure less than 90 mmHg (6.03 ± 1.13). However, based on the results of the Mann-Whitney U test, there was no significant relationship between systolic blood pressure (P = 0.948) and diastolic blood pressure (P = 0.237) and serum uric acid level (Table 3).
Based on the results of the Mann-Whitney U test, the average blood sugar was higher in hyperuricemic patients (116.6 ± 39.3) compared to non-hyperuricemic patients (114.6 ± 58.6). However, this difference was not statistically significant (P = 0.28) (Table 4).
5. Discussion
In this study, conducted to investigate the relationship between hyperuricemia and hypertension in diabetic patients undergoing hemodialysis, the rate of hyperuricemia was higher in patients with high blood pressure (16.4% versus 10%), but this difference was not significant. Also, there was no significant relationship between the factors of age, gender, and duration of dialysis with the serum level of uric acid (P < 0.05). The average uric acid level was higher in patients with systolic blood pressure < 140 mmHg and also in patients with diastolic blood pressure less than < 90 mmHg, but these differences were not statistically significant. The investigation into the relationship between hyperuricemia and average blood sugar in diabetic patients undergoing hemodialysis did not show a significant difference.
Bezerra et al. (12) did not report an independent association between hyperuricemia and systemic arterial hypertension in patients with no chronic kidney disease (CKD).
Khodeir et al.'s study showed a contradictory relationship between high uric acid levels and high systolic blood pressure in Chronic Hemodialysis Patients (13). However, Banaga et al. reported that a high serum uric acid level is a predictor of high SBP and high DBP among hemodialysis patients (14).
In the study of Feig (15), it is stated that acute high blood pressure remains dependent on uric acid and independent of sodium, while chronic blood pressure becomes independent of uric acid and dependent on sodium, and the level of serum uric acid is higher in patients with blood pressure, which is consistent with the results of the present study. Also, they reported a statistically significant relationship between age and serum uric acid levels in patients with high blood pressure, which was inconsistent with the results of our study. However, no significant relationship was reported between the serum level of uric acid and high blood pressure according to gender, which was consistent with the results of the present study.
The findings of the study by Ziaei et al. showed that the amount of uric acid in patients with macroalbuminuria was higher than in others (16). The results of the study by Voelkel et al. indicated that the mean pulmonary artery pressure and cardiac output were not correlated with the mean serum uric acid level (17). The findings of the study by Naghama et al. showed that hyperuricemia is considered a factor in patients with high blood pressure (18). Additionally, they reported a statistically significant relationship between age and serum uric acid levels in patients with high blood pressure, which was inconsistent with the results of the present study. However, regarding the relationship between the serum level of uric acid and high blood pressure, according to gender, no significant difference was observed, which is consistent with the results of the present study (18). Mallat et al. showed that hyperuricemia plays a role in the development of hypertension and CKD by inducing inflammation, endothelial dysfunction, and activation of the renin-angiotensin system (19). Also, their findings showed that the relationship between hyperuricemia and hypertension is greater in elderly patients, which was inconsistent with the results of the present study, explained by the difference in the age ranges of the samples in the two studies. Regarding the examination of the relationship based on gender, no significant differences were observed, which was consistent with the results of the present study (19).
In the study of Mirzapour et al., which examined the relationship between serum uric acid and blood sugar levels in elderly diabetic patients, it was shown that there is an inverse relationship between fasting blood sugar levels and uric acid, age, and gender, which is inconsistent with the results of the present study. The reason for the disparity can also be explained by the studied population, which was different in the two studies (3). The findings of the study by Ziaei et al. did not show a significant relationship between serum uric acid and macroalbuminuria and age in the elderly, which is consistent with our study among patients with chronic kidney failure and undergoing dialysis (16). In the study of Voelkel et al., there was no correlation between hyperuricemia and severe pulmonary hypertension according to age and gender (17).
The higher level of hyperuricemia in patients with high blood pressure has also been confirmed in other studies. It has been stated that renin-aldosterone-angiotensin stimulation leads to an increase in systolic and diastolic blood pressure in patients with high blood pressure with increased uric acid. Hyperuricemia has been found (16) and has been introduced as a predictive factor of hypertension in patients with systemic hypertension (18). Also, a significant relationship between pulmonary artery blood pressure, pulmonary vascular resistance, and cardiac output with serum uric acid level has been reported (20), and hyperuricemia has been considered effective in causing systolic blood pressure and CKD (19).
In the present study, the average blood sugar was higher in hyperuricemic patients, but this difference was not significant. This is while in Mirzapour et al.'s study, an inverse relationship between fasting blood sugar level and uric acid was observed in elderly diabetic patients (3).
However, a U-shaped pattern between serum uric acid levels and all-cause mortality among hemodialysis patients was reported (21). On the other hand, the coexistence of DM with hyperuricemia has been demonstrated to have a synergistic effect and increase the risk of mortality in patients with CKD (22). Hyperuricemia plays a role in the progression of diabetes, which involves inhibition of the insulin pathway, endothelial dysfunction, inflammation, oxidative stress, thrombus formation, and activation of the renin-angiotensin-aldosterone system, as well as chronic complications (23). In addition, the high level of uric acid creates an inflammatory state that reduces insulin sensitivity, blood glucose uptake and metabolism, also reducing the insulin production from pancreatic islet cells (24). Therefore, early diagnosis of hyperuricemia in type 2 diabetic patients and increasing awareness of lifestyle modification and healthy behavior seem to be necessary (25). Also, using hemodialysis alone for 2 sessions per week has a moderate efficacy on uric acid clearance in CKD patients; improving the Kt/V (> 1.2), and combined hemodialysis is recommended for uric acid lowering drugs and diet modifications to increase its efficacy (26). Therefore, to achieve more accurate results, it is recommended to conduct controlled studies with a larger sample size and take into account the role of other risk factors such as body mass index, the effect of smoking, alcohol, type of diets, Hb1Ac, and inflammatory index in other groups and other regions.
It should be considered that this study has some limitations. The main limitation of this study is the cross-sectional nature of the data and not showing a temporal relationship between serum uric acid levels and diabetes over time. Additionally, due to the small size of the sample, it was not possible to make a comparison based on the antidiabetic drugs used by the patients and the inflammatory index.
5.1. Conclusion
In this study, hypertension was present in 85.9% of patients. The average uric acid level was 5.9, and hyperuricemia was observed in 15.5% of patients. The analytical results of the study showed that there was no significant relationship between hyperuricemia and high blood pressure, although the rate of hyperuricemia was higher in patients with high blood pressure. In the investigation of the relationship between the average uric acid level and demographic factors such as age, sex, duration of dialysis, and blood sugar, no significant associations were found. Therefore, due to the lack of significance in this relationship, there is a need to conduct studies with a larger sample size to truly understand its association with high blood pressure in diabetic patients undergoing hemodialysis.