1. Background
Providing optimal care for diabetic patients requires continuous monitoring and evaluation of blood glucose levels (1). In these patients, due to the use of insulin, there is a possibility of a reduction in blood glucose levels and hypoglycemia (2). Severe hypoglycemia can lead to seizures, coma, and even death (3). As the duration of the disease increases, the lack of proper treatment and control of blood glucose levels will cause several side effects for the patient and threaten their health, including the risk of cardiovascular disease, hypertension, kidney problems, visual impairment, and neuropathy (4, 5). However, significant challenges remain in obtaining accurate glucose levels, including changes in environmental parameters such as temperature, skin contamination, sporadic sampling without iontophoretic stimulation, low production rates, and mixing of new and old samples (6).
There are several methods for measuring blood glucose. The gold standard method is laboratory results of venous glucose by an analyzer (7). Meanwhile, measuring capillary blood glucose with a glucometer is another method of monitoring blood glucose levels that has been widely considered. Using a glucometer is easy, and patients can use this technique to measure blood glucose at home. High speed, low cost, and easy portability are important advantages of glucometers. Furthermore, glucometer results are very helpful in regulating insulin doses and clinical management of the patient (8, 9).
Continuous blood glucose monitoring is the basis of diabetes management, and self-monitoring with a glucometer has made a significant difference in the treatment of diabetic patients and the management of this disease (10). Glucometers use the glucose oxidase reaction, and various factors such as temperature and humidity can affect the measurement of glucose and cause errors (11).
The American Diabetes Association recommends that the difference between glucometer results and laboratory results be a maximum of 15% (12). Recently, the Clinical & Laboratory Standards Institute (CLSI) recommended that for blood glucose levels less than 100 mg/dL, in 95% of the results, the difference between the glucometer results and the laboratory results should be less than 12 mg/dL, and for glucose levels above 100 mg/dL, the difference should be less than 12.5% (13).
Despite recent advances in the standardization and accuracy of glucometers, there are still problems in measuring blood glucose with this method (14). In addition to the accuracy of the device itself, the preparation of the finger for blood collection is very important. Laboratory standards recommend that hands be thoroughly washed and dried with soap and water before piercing the finger with a lancet. This is often not possible in hospitalized patients, where nurses clean the finger with an alcohol-soaked swab. While this method effectively kills germs, it has drawbacks (15).
Despite the recommendation of the World Health Organization (WHO) that after cleaning an area with a 70% alcohol-soaked swab, one should wait 30 seconds for the alcohol to dry (16), it is sometimes observed that nurses or patients at home pierce the site immediately after disinfection. This may cause alcohol to interfere with glucose measurement and dilute the blood. Some literature also recommends discarding the first drop of blood and using the second drop after disinfection (17). There are few studies on the effect of alcohol in swabs on blood glucose. For example, Ferretti and Martin reported that alcohol lowered blood glucose, while Stein showed that alcohol increased blood glucose (18, 19). Therefore, in this study, we set out to examine the effect of alcohol in swabs on blood glucose.
2. Methods
The present study is a quasi-experimental study, and the sampling method is random sampling. The required sample size was obtained at a 95% confidence level, 84% test power, test error of 3.3 (d = 3.3), and according to the results of the study by Kalatehjary et al. (S = 4.80), after quantification in the formula, 160 was obtained (20). This study was approved by the ethics committee of Birjand University of Medical Sciences (IR.BUMS.REC.1399.414). Patients were informed about the study, and all participants signed an informed consent form. We included only participants whose diabetes status had been confirmed by a physician and for whom there was information in the patient’s hospital records to confirm the diagnosis. Only those who had consented and were available to participate were included in the research. Demographic information, including age, sex, degree, and comorbidities, was collected. Blood glucose of all participants was measured in three ways: First method: Capillary blood glucose immediately after cleaning with a 70% isopropyl alcohol (AriaTeb company) swab, second method: Capillary blood glucose 30 seconds after alcohol drying, third method: Measurement of venous blood glucose.
In this study, a glucometer (GM110 model, Bonier Company) was used. Two non-dominant fingers of the patient were disinfected with a 70% alcohol pad without warming, using compression. Blood was taken from one finger immediately after disinfection, and from the other finger 30 seconds after disinfection. The patient's blood glucose was checked and recorded by the researcher. At the same time, the patient's venous blood glucose was checked and recorded for comparison. Statistical tests were used for inferential statistics, and the Kolmogorov-Smirnov test was used to check the normality of the data. In all tests, a significance level of less than 0.05 was considered. Additionally, the ANOVA test was used to compare the studied groups.
3. Results
In this study, 160 diabetic patients were included. The mean age of participants was 64.73 ± 17.81 years, with a range of 14 - 95 years. The duration of the disease was 9.39 ± 8.47 years, with a range of 1 - 30 years. More than half of the patients were male, 82 (51.2%), and most of the subjects had undergraduate education, 128 (78.1%). Results of this study showed that most of the patients were recovering, 136 (85%), while only 24 (15%) of the patients had died. Findings showed that the highest comorbidities were hypertension, 80 (50%), followed by cardiovascular disease, 51 (31.3%), and the lowest comorbidity was gastrointestinal disease, 2 (1.3%).
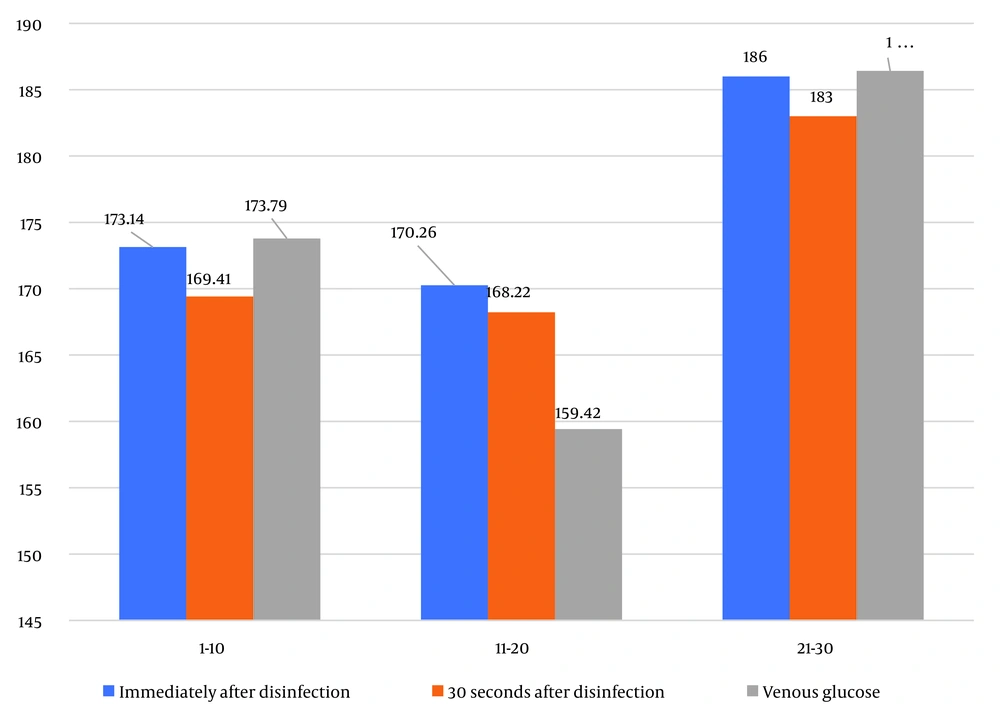
Numerical indicators of blood glucose in diabetic patients at different times—immediately after disinfection with alcohol, 30 seconds after drying of the disinfection site, and using the intravenous method—are shown in Table 1 and Figure 1.
As can be seen, the results of the ANOVA test showed no statistically significant difference between glucose measured by glucometer immediately after disinfection, blood glucose 30 seconds after disinfection, and venous glucose in the age range of 1 - 20 years (P = 0.903), in the age range of 11 - 20 years (P = 0.792), and in the age range of 21 - 30 years (P = 0.993); the results were almost the same.
Additionally, the results of the ANOVA test showed no statistically significant difference in glucose measured by glucometer immediately after disinfection between the three periods of time (P = 0.831). Similarly, no statistically significant difference was observed in blood glucose 30 seconds after drying between the three periods of time (P = 0.826). Furthermore, no statistically significant difference was observed in venous glucose between the three periods of time (P = 0.410).
| Disease Duration Blood Glucose | 1 - 10 (y) (n = 98) | 11 - 20 (n = 50) | 21 - 30 (n = 12) | Total (n = 480) | P a |
|---|---|---|---|---|---|
| Immediately after disinfection | 173.14 ± 74.65 | 170.26 ± 91.74 | 186.00 ± 74.78 | 173.21 ± 79.97 | 0.831 |
| 30 seconds after drying | 169.41 ± 71.47 | 168.22 ± 84.48 | 183.00 ± 75.92 | 170.06 ± 75.69 | 0.826 |
| Venous glucose | 173.79 ± 73.96 | 159.42 ± 75.71 | 186.42 ± 86.81 | 170.24 ± 75.43 | 0.410 |
| P a | 0.903 | 0.792 | 0.993 | 0.919 |
a ANOVA.
b Values are expressed as Mean ± SD.
4. Discussion
Results of the study showed that there was no significant difference between the three methods. In a study conducted by Dunning et al. (21), blood glucose of 911 patients was measured with a glucometer in two ways: Before evaporation of alcohol and after evaporation of alcohol. Results indicated that there was no significant difference between the two methods. Evaluation of the interfering effect of alcohol in vitro has shown that after mixing blood and alcohol, alcohol levels above 10% strongly affect blood glucose (22). In another study, Mahoney et al. examined the effect of alcoholic swabs on glucose measured with a glucometer. Results of this study were in line with our study, showing no significant difference in blood glucose levels between the two methods: Immediately after disinfection and after alcohol evaporation (23).
Foos measured glucose with a glucometer in four ways using contour next test strips based on glucose hydrogenase. The results of this study showed that in groups that did not use alcohol, there was no difference in blood glucose levels between the first and second drops. However, in the groups that used alcohol for disinfection, the blood sugar level in the first drop was 2.1 mg lower than the second drop. This difference was small and not significant (24).
Most recently, in 2021, a study conducted by Jonca et al. involved 50 participants who were divided into four groups. The study measured blood glucose in different conditions: Washing hands with soap and water and measuring blood glucose immediately after washing and 30 seconds after drying hands, and disinfecting hands with alcohol and taking blood immediately after disinfection and 30 seconds later. In groups 3 and 4, the conditions were the same as in groups 1 and 2, but the type of lancet used was different and was thinner. The results showed that although alcohol affected the results, the difference was not significant. The authors also investigated the effect of lancet thickness on the interference of alcohol (25).
Typically, 21G lancets are used in hospitals for capillary blood collection, removing 32.5 microliters of blood from the finger. In contrast, patients at home usually use 30G lancets, which remove 20 microliters of blood. They found that when using the 30G lancet, blood glucose was lower when taken immediately after alcohol disinfection compared to 30 seconds after disinfection. The results suggested that when thinner lancets are used, the volume of blood is less, causing the alcohol to dilute the blood and reduce the measured blood glucose (25).
4.1. Conclusions
The results of our study showed that alcohol-containing swabs used for hand disinfection do not have a significant effect on the measured blood glucose, although it is better to wait 30 seconds for the alcohol to dry, especially when thin lancets are used. Due to the limitations of our study, it is recommended that future studies be conducted with a larger statistical population and different sample sizes.