1. Background
Colorectal cancer (CRC) is the third most common cancer worldwide (1). Despite advancements in CRC diagnosis and treatment, the number of cases continues to rise (2). Cell death is a crucial process for development, homeostasis, and response to external stimuli, with several types, including apoptosis, necrosis, autophagy, and pyroptosis. Apoptosis eliminates damaged or unnecessary cells, whereas necrosis involves rapid cellular swelling and membrane rupture. Dysregulated autophagy can lead to cell death, and pyroptosis is an inflammatory form of cell death. The p53 tumor suppressor gene, which is frequently altered in CRC, plays a key role in regulating DNA repair, the cell cycle, senescence, metabolism, and cell death in response to stress signals such as DNA damage (3).
Apoptosis is initiated via two primary pathways: The intrinsic (mitochondrial) pathway and the extrinsic pathway, which involves death receptors (4). The intrinsic pathway is regulated by Bcl2 family proteins, which control mitochondrial outer membrane permeabilization and trigger cytochrome c release in response to intracellular stress (5). Once released into the cytosol, cytochrome c binds to APAF1, leading to its oligomerization and the activation of caspase-9. Activated caspase-9 subsequently triggers the cleavage and activation of caspases-3 and -7, facilitating apoptosis through the degradation of cellular proteins (6).
The use of bioactive compounds from plants has gained attention for their potential to inhibit tumorigenesis and induce apoptosis in cancer cells. Herbal medicines are of particular interest due to their ability to reduce chemotherapy-related side effects and offer alternative therapeutic options (7, 8). Artemisia annua, commonly known as sweet wormwood, is one such herbal medicine (9). Recent studies indicate that artemisinin, a compound found in A. annua, not only has anti-inflammatory properties but also induces apoptosis in cancer cells (10). The leaves of A. annua are long and narrow, measuring approximately 3 - 5 cm in length and 0.2 - 1 cm in width, with a bright green, hairy appearance (11).
The primary form of A. annua used for medicinal purposes is tea, which has historically been used to treat fevers, including malaria. The leaves of the plant are the source of its therapeutic properties, known for their potent antioxidant and anticancer capabilities. Artemisia annua is rich in a diverse array of compounds, including essential oils, polysaccharides, saponins, coumarins, acids, minerals, flavonoids, polyphenols, and artemisinin. These compounds contribute to its use in the treatment and prevention of cancer and malaria. Research highlights the safety and medicinal potential of A. annua leaf preparations, even when artemisinin levels are low (12, 13). Studies have confirmed the anticancer properties of A. annua extracts against the MDA-MB-231 cell line (14) and their inhibitory effects on Reh and Nalm-6 cell lines (15). Additionally, artemisinin has been shown to inhibit the growth of human gastric cancer cells and induce apoptosis (16).
2. Objectives
While previous studies have demonstrated the anticancer effects of A. annua on various cell lines, its impact on HT-29 colorectal cancer cells remains unclear. This study aims to investigate the cytotoxicity of A. annua extract and its modulation of the P53 and BAX/BCl2 ratio in HT-29 cells.
3. Methods
3.1. Materials
The cell culture medium (DMEM, Code No: BI-1003), fetal bovine serum (FBS), penicillin-streptomycin (Code No: AC16-025), and DMSO (Cat No: 196055) were purchased from Bioidea.co (Tehran, Iran). The HT-29 cell line was obtained from the Iranian Biological Resource Center (Tehran, Iran). MTT (Cat No: M5655), [propidium iodide (PI), Code No: P4170)], and Tris-base (Cat No: 108382) were sourced from Sigma Chemical Co. (St. Louis, MO, USA). Kiazol reagent (Cat No: KZOL50) was purchased from Kiazist.co (Hamedan, Iran). The cDNA synthesis kit (Cat No: YT4500) from Favorgen (Taiwan) and RNase A (Cat No: YT9055) were obtained from Yektatajhiz.co (Tehran, Iran). SYBR Green Master Mix (Catalog Number: A325402) was sourced from Amplicon (Denmark).
3.2. Preparation of Methanolic Extract
Artemisia annua leaves were collected from the suburbs of Galikesh (Golestan province, Iran). The plant was authenticated at the International Herbarium Center of Ferdowsi University of Mashhad, Iran, and a voucher specimen (No. 1347) was deposited in the herbarium. The leaves were separated, washed, and dried at room temperature, then ground into powder, keeping it away from direct sunlight. The powder was mixed with 100 mL of 96% methanol, placed on a shaker for 72 hours, and filtered through Whatman cellulose filter papers (No. 1) to remove any debris. The solution was then concentrated using a rotary evaporator (Heidolph, Germany) set at 40°C. The concentrated extract was used to prepare methanolic extract in various doses.
3.3. Cell Culture
HT-29 cells were cultured in DMEM medium supplemented with 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin. The cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
3.4. Cell Viability Assay
After 3 passages, the cells were exposed to various concentrations (20 - 2000 μg.mL-1) of A. annua methanolic extract and 4 μM (or 2.17 μg.mL-1) Doxorubicin as a positive control, with each concentration tested in triplicate. The cytotoxic effect was assessed at 24 and 48 hours using the MTT assay. Following treatment, the culture medium was replaced with a 10% MTT solution, and the absorbance was measured at 570 nm and 630 nm using an ELISA reader (BioTek Instruments, USA).
3.5. RNA Extraction, Reverse Transcription-PCR, and qReverse Transcription -PCR
qreverse transcription (RT)-PCR was used to analyze the expression of the p53, BAX, and Bcl2 genes. Cells were treated with Doxorubicin and A. annua extract. After 48 hours, RNA was extracted using Kiazol reagent, and its purity was assessed using a Nanodrop spectrophotometer. The RNA purity was determined by measuring absorbance at 260 and 280 nm, calculating the 260/280 ratio. cDNA was synthesized using a cDNA synthesis kit. After primer design, qRT-PCR was performed using a StepOne™ real-time PCR system (Thermo Fisher Scientific, USA). The real-time PCR procedure included the following steps: Initial heating at 50°C for 2 minutes, denaturation at 95°C for 1 minute, followed by 40 cycles of denaturation at 95°C for 15 seconds, annealing at 60°C for 45 seconds, and extension at 72°C for 45 seconds. The primer sequences used for qRT-PCR are listed in Table 1. β-Actin was used as the reference gene for normalization. The relative expression levels of p53, BAX, and Bcl2 were calculated using the 2-ΔΔcT method.
| Target Gene | Primer Sequence (5′ to 3′) |
|---|---|
| BAX | |
| Forward | 5'CCCGAGAGGTCTTTTTCCGAG3' |
| Reverse | 5'CCAGCCCATGATGTTCTGAT3' |
| Bcl2 | |
| Forward | 5'GGTGGGGTCATGTGTGTGG3' |
| Reverse | 5'CGGTTCAGGTACTCAGTCATCC3' |
| p53 | |
| Forward | 5'AGGGTTAGTTTACAATCAGC3' |
| Reverse | 5'GGTAGGTGCAAATGCC3' |
| Beta-actin | |
| Forward | 5'CATGTACGTTGCTATCCAGGC3' |
| Reverse | 5'CTCCTTAATGTCACGCACGAT3' |
3.6. PI Staining
Cells (700,000) were treated with 750 μg.mL-1 of A. annua extract and 5 μM (2.71 μg.mL-1) of Doxorubicin for 48 hours. Following treatment, the cells were fixed in 70% ethanol and stored at -20°C overnight. After centrifugation, the cells were resuspended in 1X binding buffer, treated with RNase A, and stained with PI. The samples were then incubated in the dark. Apoptosis induced by the extract was quantified using a flow cytometer (Sysmex, Japan) and analyzed with FlowJo software (v7.6.1). Apoptotic cells were identified by positive PI staining, and 15,000 single cells were measured per technical replicate after doublet cells were excluded using an FSC-H and FSC-A scatter plot.
3.7. Statistical Analysis
Calcusyn and SPSS version 19 software were used to determine and compare half-maximal inhibitory concentration (IC50) values. Additionally, for real-time PCR data analysis, SPSS version 19, Excel, and REST software were employed. Statistical analysis was conducted using a one-way ANOVA test. GraphPad Prism version 9 was used to generate graphs for visual representation of the results. Values are presented as the average of three separate determinations, shown as mean ± SD. Significant differences were considered at the level of P < 0.05.
4. Results
4.1. Inhibitory Effect of the Methanolic Artemisia annua Extract Against Cancer Cells
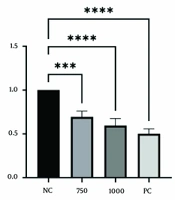
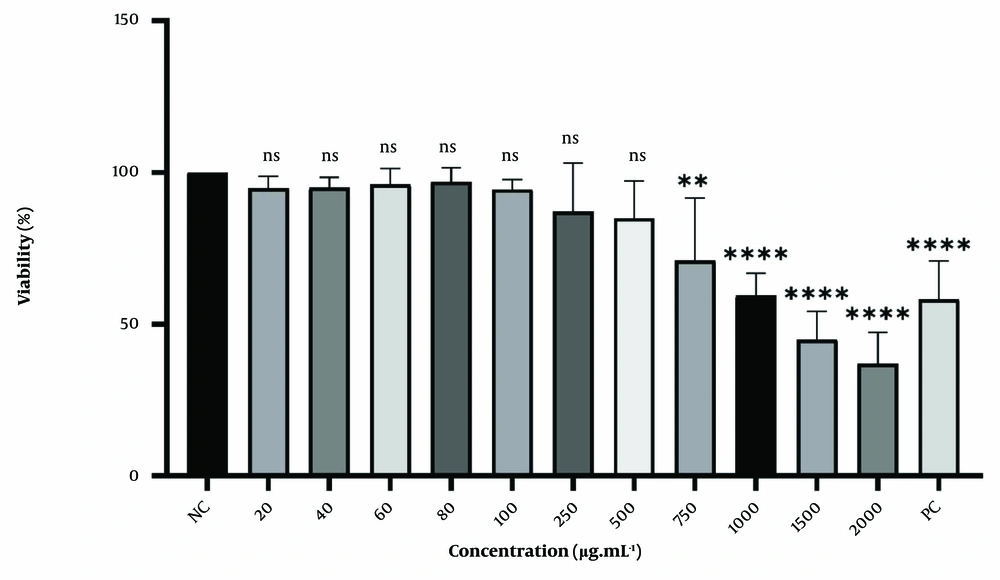
The cell viability and morphological changes of HT-29 cells treated with varying concentrations of A. annua methanolic extracts were measured to determine the extract's impact on the growth of human colorectal cancer cells. Cytotoxicity was assessed using an MTT assay. Figure 1 demonstrates a concentration-dependent decrease in cell viability in HT-29 cells after 48 hours of treatment. Low concentrations (ranging from 20 to 500 μg.mL-1) did not result in significant changes, though a slight decrease in viability (approximately 20%) was noted. As the concentration increased, the reduction in viability became more pronounced. After 48 hours, cell viability decreased to 36% when treated with a 2000 µg.mL-1 concentration of A. annua extract.
Cytotoxic effect of A. annua methanolic extract and Doxorubicin on HT-29 cells. Cells were treated for 48hours. All data are shown as mean ± SD. NC, negative control: Cells with no treatment; PC, positive control: Cells treated with 4 μM concentration of Doxorubicin. ns, non-significant; ** P < 0.01; **** P < 0.0001, compared with control.
4.2. Apoptotic Effect of Methanolic Artemisia annua Extract Against Cancer Cells
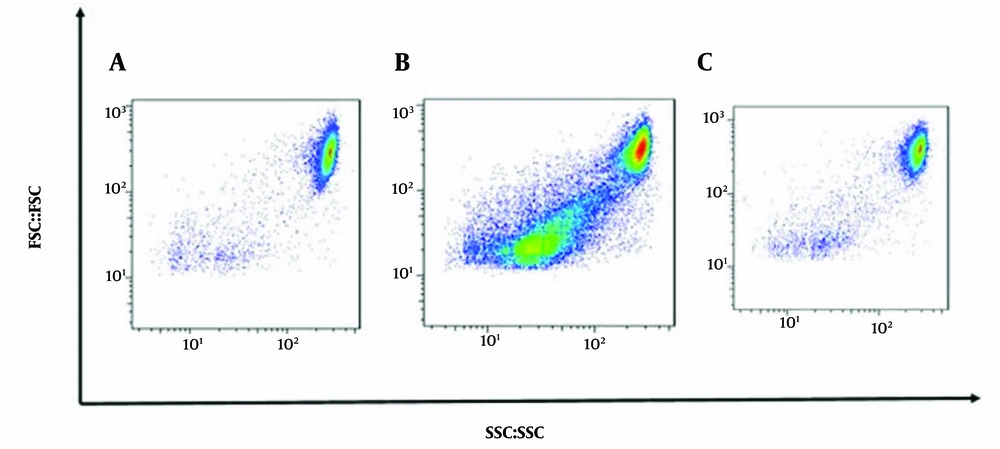
To further explore the mechanisms underlying A. annua -induced cell death, DNA content analysis was performed using flow cytometry on HT-29 cells treated with A. annua extract. As anticipated, treatment with A. annua extract led to a significant, time-dependent decrease in the cell population in the S and G2/M phases compared to the control. This reduction was accompanied by an increase in the cell population in the sub-G1 phase, indicating cell death (Figure 2).
4.3. Cell Cycle Analysis of Cancer Cells Treated with Artemisia annua Extract
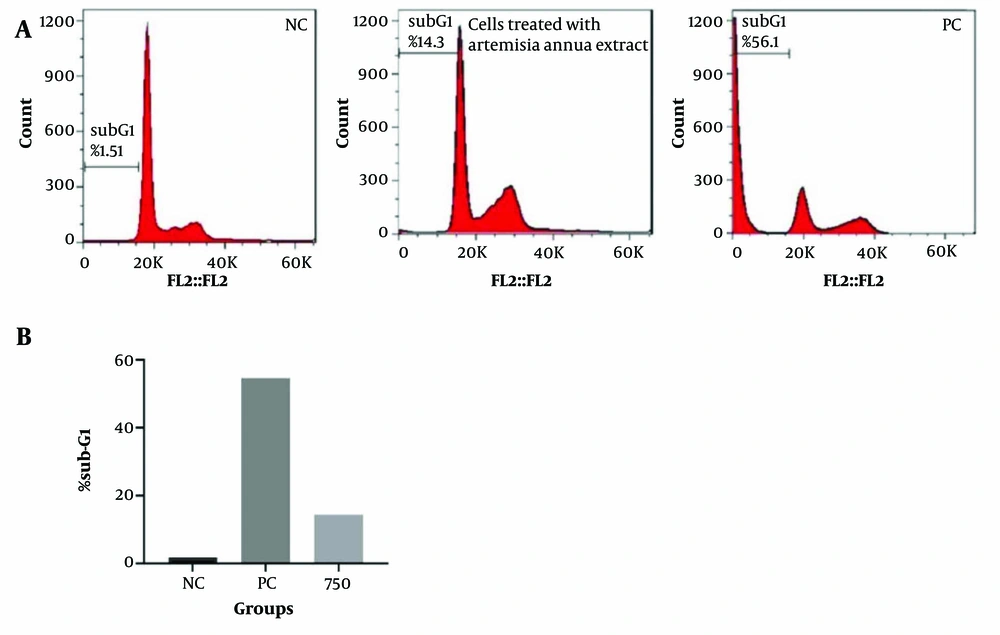
Since cell cycle arrest is a critical step in inhibiting cell growth and inducing cell death, the cell cycle distribution of the A. annua extract-treated cells was evaluated. As anticipated, the A. annua extract significantly arrested tumor cells in the G1 phase (P < 0.05). Additionally, when HT-29 cells were treated with 750 μg.mL-1 of A. annua extract and 4 μM of Doxorubicin, an increase in the cell population in the subG1 phase was observed after 48 hours (Figure 3).
A, Artemisia annua extract induced cell cycle arrest in HT-29 cells treated with Artemisia. NC, negative control group: Cells treated with a concentration of 5 μM of Doxorubicin; B, percentage of cells in sub-G1 phase. NC, negative control group; PC, positive control: Cells treated with a concentration of 5 μM of Doxorubicin.
4.4. qRT–PCR Analysis of the Genes Involved in Apoptosis
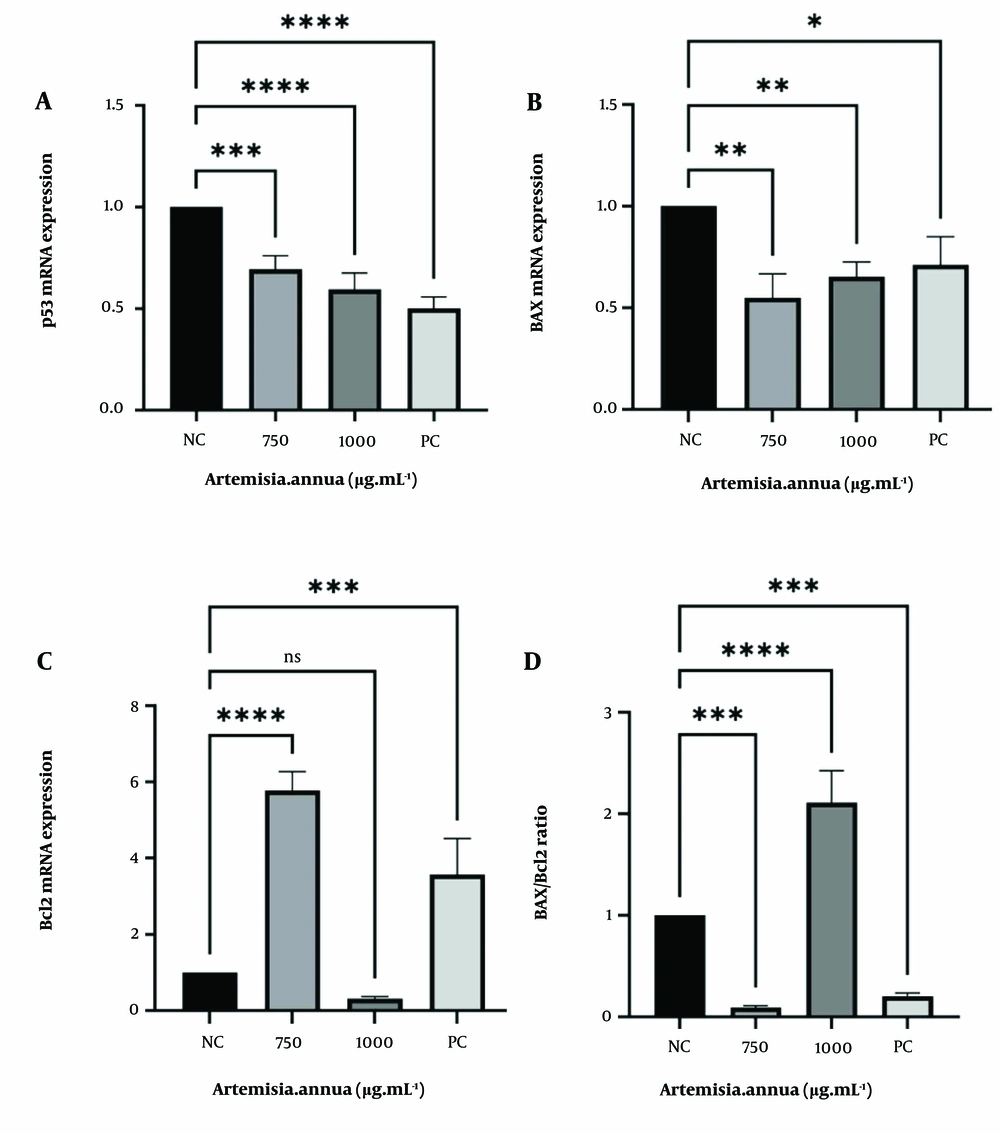
As shown in Figure 4, the expression of p53 significantly decreased after treatment with 750 and 1000 μg.mL-1 concentrations of A. annua extract for 48 hours. Additionally, the figure illustrates a 5-fold up-regulation of Bcl2 after treatment with 750 μg.mL-1 of A. annua extract. However, when the cells were treated with 1000 μg.mL-1, no significant change in Bcl2 mRNA expression was observed compared to the control. We also examined the BAX/Bcl2 ratio after A. annua extract treatment, which revealed a sharp decrease in the ratio following exposure to 750 μg.mL-1.
A, p53; B, BAX; and C, Bcl2 mRNA expression were assessed by quantitative reverse transcription (RT)-PCR. Cells were treated for 48hours, and mRNA level was used for evaluating gene expression. The normalization process involves using the expression level of β-Actin as a reference. NC, negative control: Cells with no treatment; PC, positive control: Cells treated with 4 μM concentration of Doxorubicin. ns, non-significant; * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001 compared with control.
5. Discussion
Colorectal cancer remains a significant global health issue with high mortality rates despite advancements in treatment (17). Numerous studies have demonstrated that compounds derived from medicinal plants hold valuable anticancer potential and can modulate drug resistance in cancer therapy. Artemisia annua, traditionally used to treat various health conditions, is one such plant with promising anticancer properties. Although artemisinin is recognized as a key compound, other components of A. annua may also contribute to its anticancer effects, though further clinical trials are necessary to validate these findings (18). A case study by Michaelsen et al. documented reduced PSA levels in a prostate cancer patient following long-term use of A. annua capsules, with a regimen of 5 capsules daily, each containing 50 mg (19).
The current study revealed that the methanolic extract of A. annua inhibits the proliferation of HT-29 cells in a time- and dose-dependent manner. Previous studies have shown that 70 μg/mL of methanolic extract of A. annua reduced cell viability in Reh and Nalm-6 cell lines by 52% after 48 hours and 47% after 72 hours. In contrast, our study demonstrated that 1000 μg/mL reduced cell viability to 59%, and 1500 μg/mL reduced viability to 44% after 48 hours. Variations in these results can be attributed to differences in species or cultivars of the plant, extraction conditions, and the timing of extraction (20). Emami et al. found that A. annua extracts, particularly methanolic extracts, induced primary and secondary apoptosis in AGS cell lines, with an IC50 value of 500 μg.mL-1 (21).
Additionally, numerous studies have confirmed that dihydroartemisinin can arrest the cell cycle in the G1 phase, induce apoptotic cell death, and elevate ROS levels (22). A recent study by Lin et al. demonstrated that artemisinin exhibited significant toxicity in the HCT-116 cell line (23). Studies also show that p53 is a crucial tumor suppressor gene (24). BAX promotes apoptosis by permeabilizing the mitochondrial outer membrane in response to stress, while Bcl2 blocks apoptosis (25, 26). Lu et al. found that dihydroartemisinin induced G1 cell cycle arrest and increased the BAX/Bcl2 ratio (27). Another study revealed increased mRNA expression of BAX and decreased Bcl2 in Nalm-6 and Reh cell lines when treated with 40 μg.mL-1 of A. annua extract (20). However, in our study, cells treated with 750 μg.mL-1 showed substantial up-regulation of BAX and down-regulation of Bcl2.
This study investigated the cytotoxic effects of methanolic A. annua extract on the HT-29 cell line and analyzed the relative changes in p53, BAX, and Bcl2 gene expression. Morphological changes were also evaluated after treatment with the IC50 concentration of the methanolic extract. The results indicated a decrease in p53 gene expression and a 2-fold increase in the BAX/Bcl2 ratio following exposure to 1000 μg.mL-1. Flow cytometry confirmed the cytotoxic effects of the extract. However, despite the changes in apoptotic and pro-apoptotic genes, the analysis suggests that cell death induced by the extract is not primarily due to apoptosis.
5.1. Conclusions
The findings from the MTT and PI assays confirm the cytotoxic effects of the extract on HT-29 cells. However, the gene expression analysis of BAX, Bcl2, and p53 suggests that the observed cell death is not primarily driven by apoptosis.