1. Background
More than 15 million preterm infants are born worldwide each year, and the morbidity and mortality rates of preterm neonates (gestational age ≤ 37 weeks) are much higher than those of term neonates (1). The risk of mortality due to prematurity is 28%, and only 37% of preterm infants have a chance of surviving without neonatal morbidity. Respiratory disorders such as respiratory distress syndrome (RDS), bronchopulmonary dysplasia (BPD), pneumothorax, cardiovascular, hematologic, and renal complications, gastrointestinal complications including necrotizing enterocolitis (NEC), direct and indirect hyperbilirubinemia, metabolic-endocrine complications (hypocalcemia, hypoglycemia, hyperglycemia), central nervous system complications such as intraventricular hemorrhage (IVH), sepsis (early and late), and other infections are significant medical morbidities in preterm infants (2). Feeding intolerance, postnatal growth difficulties, neurodevelopmental problems, and metabolic diseases are other complications in preterm neonates (3).
The highest risk associated with indirect hyperbilirubinemia is kernicterus (bilirubin encephalopathy), which presents with high levels of indirect bilirubin and symptoms indistinguishable from early sepsis, asphyxia, or hypoglycemia. The goal of treating hyperbilirubinemia is to prevent this neurotoxicity; hence, phototherapy and blood transfusions are the main treatments. Intravenous immunoglobulin (IVIG) and metalloporphyrins are other treatment options (1-5). The most common gastrointestinal emergency complication in neonates is NEC, which is life-threatening (1, 6, 7). Unfortunately, medical morbidity and premature neonate mortality impose significant costs on health systems (3), making it essential to use strategies that can reduce the risk of mortality, treatment costs, and hospitalization time in preterm infants.
In this regard, the prophylactic use of oral probiotics has been recently suggested. Probiotics are living organisms that have beneficial effects on the health of their consumers (8, 9). Probiotics are isolated from human microbiota and consumed to increase the level of beneficial organisms present in the microbiota (1-5)-(6-10). Probiotics have been shown to reduce the risk of NEC, mortality, sepsis, and feeding intolerance in very low birth weight (VLBW) neonates. They exert these effects by strengthening the intestinal barrier, modulating the immune response, and directly inhibiting intestinal colonization by pathogens (11-14). In many developed countries, probiotics are now commonly used in preterm neonates to prevent NEC (1).
Synbiotics are combinations of a probiotic and a prebiotic (prebiotics are carbohydrates such as oligosaccharides that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, thereby improving host health) (1). The aim of this study was to compare the effect of two drugs containing probiotics: Pedilact® (synbiotic) and Reuteflor® (probiotic) on important health outcomes including mortality, NEC, late-onset sepsis, weight gain, neonatal icterus, hospitalization time, etc., in preterm infants admitted to the NICU of Valiasr Hospital in Birjand.
2. Objectives
The aim of this study is to find a way to reduce mortality risk, decrease the length of hospital stay, and lower hospital-acquired infections due to prolonged hospitalization, as well as reduce treatment costs. This is important because if probiotics can reduce the complications of prematurity, leading to a shorter hospital stay, it can ultimately have a positive impact on neurodevelopmental progress, intellectual development, physical growth indicators, and the prevention of chronic pulmonary and gastrointestinal issues in the later years of the child's life.
3. Methods
3.1. Study Design
This study is a double-blind clinical trial.
3.2. Participants
The study population consisted of premature neonates weighing 1000 - 2000 g who were admitted to the NICU of Valiasr Hospital in Birjand, from June to October 2021.
3.3. Scales
The sample size was calculated based on previous studies (11). Using the mean time to reach full enteral feeding in the probiotic group (12.83 ± 4.26 days) and the placebo group (16.75 ± 6.59 days), and according to the formula for comparing means in two groups with 95% confidence and 80% power, 30 neonates per group were calculated.
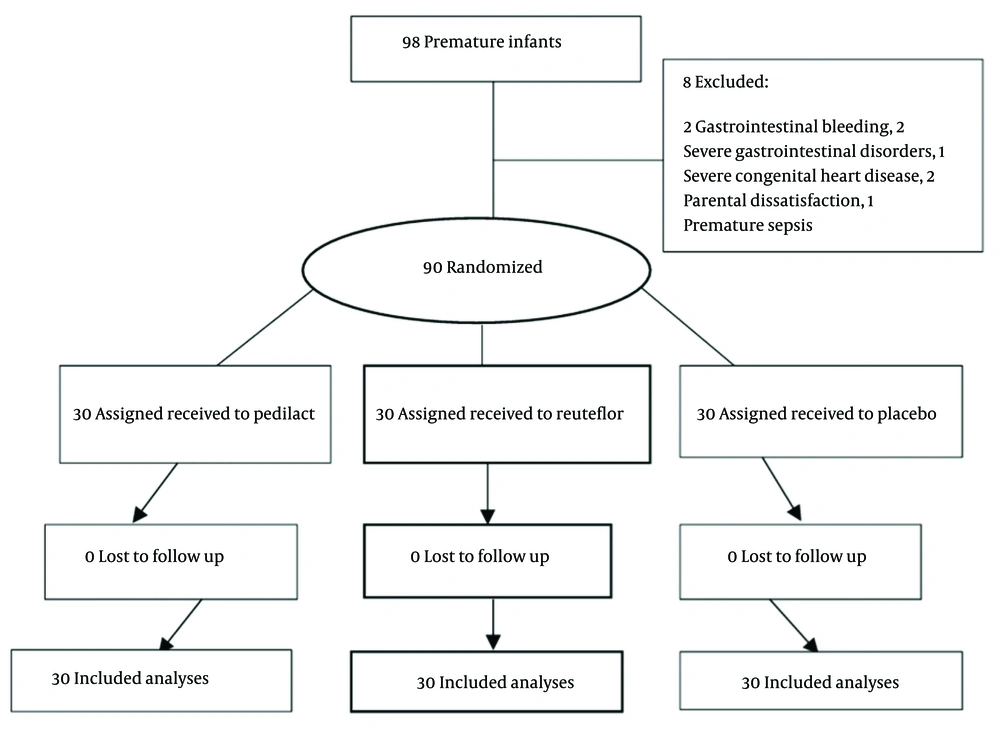
Initially, 98 neonates were selected by a non-random sampling method (available), who met the inclusion criteria (weight 1000 - 2000 g admitted to the NICU). The steps of selecting and allocating patients are shown in Figure 1.
3.4. Data Collection
Secondly, all the neonates’ parents were informed about the aims of the project, complications, and possible risks associated with the use of the drugs. Thirdly, after obtaining written consent from the parents, the neonates were included in the study. Exclusion criteria included parental dissatisfaction, disorders such as gastrointestinal obstruction, gastrointestinal bleeding, emphysema, high-grade heart disease, ventilation for more than 24 hours, asphyxia, IVH, early sepsis with positive culture, and any factor that caused the patient to become NPO (nothing by mouth) for over 24 hours during the treatment. As a result, eight neonates were excluded from the study: Two neonates with gastrointestinal bleeding, two neonates with severe gastrointestinal disorders (duodenal obstruction and atresia), one neonate with premature sepsis, one neonate due to severe congenital heart disease, and two neonates due to hospital discharge (personal satisfaction).
The feeding phase was initiated by giving the babies five drops of the assigned drug daily for one week. The drug was provided in identical jars marked with a code. The first intervention group received Pedilact drops from ZistTakhmir Company, made in Iran, containing Lactobacillus reuteri, L. rhamnosus, and Bifidobacterium infantis at 109 CFU. The second group received Reuteflor drops from Faradaro and Fanvarmehr Company, made in Iran, containing L. reuteri at 8 × 108 CFU. The third group received distilled water.
A checklist was designed for the daily evaluation of variables, including neonate weight, study group, gestational age, sex, demographic characteristics of the mother and neonate, feeding start time, time to reach full enteral feeding (volume of milk reaching 120 cc/kg/day), incidence of NEC and jaundice, mortality and late-onset sepsis, mother and neonate blood group, number of days of hospitalization, phototherapy, oxygen therapy, and weight at the time of neonatal discharge.
3.5. Data Analysis
Data were entered into SPSS 22 software and analyzed using statistical tests, including analysis of variance (or the Kruskal-Wallis test in cases of abnormal distribution) and the chi-square test or Fisher's exact test, with a significance level of α = 0.05.
3.6. Ethical Consideration
Considering that many studies have previously been conducted on preterm infants with probiotics, and no significant adverse effects of these medications have been reported in the study groups, parents were informed about the objectives of the study, and the potential side effects and risks associated with the use of these medications were explained to the infants' guardians. After obtaining full consent from the guardians and receiving a signed consent form, the subjects were enrolled in the study. The checklists were anonymized (without names or surnames) and analyzed in aggregate. This study was approved by the ethics committee of Birjand University of Medical Sciences (code: IR.BUMS.REC.1399.498) and was registered in the Iranian Registry of Clinical Trials (code: IRCT20210102049922N1).
4. Results
In this study, 90 preterm infants with a mean weight of 1547.19 ± 285.5 g participated, categorized into three groups (two intervention groups and one placebo group). The observations revealed that most of the neonates were boys, and the mean weight, height, head circumference, gestational age, gravidity, and maternal age were not statistically significant between the three groups (P < 0.05) (Table 1). The mean days to reach full enteral feeding in all neonates were 8.09 ± 4.04 days. Although it was longer in the placebo group compared to the groups receiving Reuteflor and Pedilact, this difference was not statistically significant (Table 2).
| Characteristic | Pedilact Group | Reuteflor Group | Placebo Group | P-Value |
|---|---|---|---|---|
| Weight (g) | 1546.30 ± 282.46 | 1532.33 ± 276.85 | 1562 ± 305.52 | 0.91 b |
| Length (cm) | 41.70 ± 3.30 | 40.43 ± 3.66 | 41.31 ± 3.49 | 0.24 b |
| Head circumference (cm) | 29.41 ± 1.83 | 28.68 ± 1.81 | 29.36 ± 1.59 | 0.20 b |
| Gestational age (w) | 32.53 ± 2.95 | 31.6 ± 1.75 | 31.93 ± 2.26 | 0.20 b |
| Gravidity | 2.80 ± 1.54 | 2.60 ± 1.42 | 2.50 ± 1.38 | 0.71 b |
| Mother age (y) | 29.23 ± 6.86 | 30.16 ± 5.89 | 28.6 ± 7.12 | 0.65 b |
| Start time of feeding (h) | 20.70 ± 10.50 | 20.40 ± 7.05 | 20.90 ± 11.60 | 0.85 b |
| Gender | 0.06 c | |||
| Male | 12 (40) | 15 (50) | 21 (70) | |
| Female | 18 (60) | 15 (50) | 9 (30) | |
| Singleton | 0.26 d | |||
| One | 22 (73.3) | 27 (90) | 20 (66.7) | |
| Two | 6 (20) | 2 (6.7) | 7 (23.3) | |
| Three | 2 (6.7) | 1 (3.3) | 3 (10) | |
| Blood group incompatibility | 1 c | |||
| Yes | 5 (16/7) | 5 (16/7) | 5 (16/7) | |
| No | 25 (83.3) | 25 (83.3) | 25 (83.3) | |
| RH incompatibility | 1 c | |||
| Yes | 2 (6.7) | 2 (6.7) | 2 (6.7) | |
| No | 28 (93.3) | 28 (93.3) | 28 (93.3) | |
| Maternal smoking | 0.32 d | |||
| Yes | 2 (6.7) | 0 (0) | 0 (0) | |
| No | 28 (93.3) | 30 (100) | 30 (100) | |
| Mother opium addict | 0.32 d | |||
| Yes | 5 (16.7) | 1 (3.30 | 4 (13.3) | |
| No | 25 (83.3) | 29 (96.7) | 26 (86.7) |
a Values are expressed as mean ± SD or No. (%).
b One way ANOVA.
c Chi-square.
d Fisher exact test.
| Outcomes | Pedilact Group a | Reuteflor Group a | Placebo Group a | P-Value b |
|---|---|---|---|---|
| Full enteral feeding (d) | 7 (4.7 & 10) | 7 (5 & 9) | 8.5 (6 & 11) | 0.06 |
| Duration of hospitalization (d) | 11.5 (9.7 & 20) | 9.5 (7 & 15.25) | 10 (8 & 15) | 0.32 |
| Duration of auxiliary oxygen requirement (d) | 2 (1 & 4) | 1.75 (1 & 3.25) | 4 (2 & 6) | 0.004 |
| Mean daily weight gain (g) | 8.25 (4.7 & 17.3) | 4.11 (-5.2 & 12.6) | 0.21 (-7.5 & 5.6) | 0.002 |
| Phototherapy duration (d) | 2 (1.8 & 2.5) | 2.25 (2 & 3) | 3 (2 & 4) | 0.001 |
a Median (25th and 75th percentile).
b Kruskal-Wallis H.
The average number of days that participants spent in the hospital in the Pedilact group was more than in the other groups, while there was no statistically significant difference in hospitalization days between the three groups (P = 0.32) (Table 2). The mean number of required days for oxygen therapy was significantly different between the study groups (P = 0.004). More days were needed in the placebo group compared to the Pedilact group (P = 0.01) and the Reuteflor group (P = 0.002). However, there was no difference between the two intervention groups (P = 0.38) (Table 2).
Furthermore, the mean total weight gain in infants was 94.56 ± 174.6 g, and the mean daily weight gain was 5.16 ± 13 g. These were significantly different among the study groups (P = 0.005), with the most weight gain and daily weight gain in the Pedilact group and the least in the placebo group. Therefore, weight gain was significant between the Pedilact and Reuteflor groups (P = 0.04) and between the Pedilact and placebo groups (P = 0.001), with no significant difference between the Reuteflor and placebo groups (Table 2).
In addition, the mean days of phototherapy in all neonates were 2.42 ± 1.15 days. There was a significant difference in mean days of phototherapy in the study groups (P = 0.001). Although there was no significant difference between the Pedilact and Reuteflor groups (P = 0.07), there was a significant difference between the Pedilact and placebo groups (P < 0.001) and the Reuteflor and placebo groups (P = 0.01) (Table 2).
Additionally, the incidence of NEC in the Pedilact group was zero, while in the Reuteflor group and placebo, it was 3.3%. Therefore, the incidence of NEC in the studied groups was not statistically significant (Table 3). It was also observed that mortality in the Reuteflor group was 3.3%, while it was zero in the other two groups. Moreover, the incidence of mortality, sepsis, and icterus in the studied groups did not differ significantly (Table 3).
| Outcomes | Pedilact Group | Reuteflor Group | Placebo Group | P-Value b |
|---|---|---|---|---|
| Icter | 27 (90) | 28 (93.3) | 27 (90) | 0.87 |
| NEC | 0 (0) | 1 (3.3) | 1 (3.3) | 1 |
| Mortality | 0 (0) | 1 (3.3) | 0 (0) | 1 |
| Sepsis | 0 (0) | 0 (0) | 0 (0) | 1 |
Abbreviation: NEC, necrotizing enterocolitis.
a Values are expressed as No. (%).
b Fisher exact test.
5. Discussion
The main aim of this study was to compare the effect of two drugs containing probiotics: Pedilact® (synbiotic) and Reuteflor® (probiotic) on important health outcomes in preterm infants. The evaluation demonstrated that the mean days to reach full enteral feeding was not statistically significant between the three groups. Our results are similar to the studies of Dehghan et al. (15), Zahed Pasha et al. (16), and Sun et al. (7). In other studies, such as Nouri Shadkam et al. (11), Cui et al. (17), and Indrio et al. (18), this period in probiotic groups was significantly shorter, which could be due to differences in the type, duration, and dose of probiotics consumption.
Our study revealed that there was no statistically significant difference in the mean days of hospitalization. Our results are similar to the study of Dehghan et al. (15) who used Pedilact. However, in some other studies performed by Indrio et al. (18), Sun et al. (7), and Zahed Pasha et al. (16), the time of hospitalization in probiotic groups was significantly shorter. This difference could be due to the type, dose, and duration of drug use in those studies.
The mean required days for oxygen therapy were significantly more in the placebo group compared to the Pedilact and Reuteflor groups. Some relevant studies have shown that the use of probiotics is effective in the prevention of BPD in preterm infants. A study by Qu et al. (19) found that receiving a probiotic containing Clostridium butyricum reduced BPD risk in infants less than 32 weeks’ gestational age, while a meta-analysis by Villamor-Martinez et al. (20) could not prove a significant effect between probiotic consumption and BPD. Probiotics with anti-infection and anti-inflammatory effects on the lungs can reduce respiratory distress and oxygen demand (21), although respiratory failure and oxygen therapy in the first days of life in preterm infants depend on various factors.
In our study, the most weight gain was in the Pedilact group and the least was observed in the placebo group. These results are similar to the studies by Sun et al. (7), Cui et al. (17), Indrio et al. (18), and the study of Zahed Pasha et al. (16). It was observed that premature infants receiving probiotics gained more weight compared to control groups, and this difference was not significant. However, the type of probiotic, dose, duration, age, and birth weight were different in these studies. It seems that combined probiotics (synbiotic) improve gastrointestinal function by increasing motility and reducing reflux, leading to better tolerance of oral nutrition (feed tolerance) and better weight gain and reduction of other gastrointestinal complications.
In this study, phototherapy days were not significantly different between the Pedilact and Reuteflor groups, but there was a significant difference between probiotics-exposed groups and the placebo group. In this study, phototherapy days were not significantly different between the Pedilact and Reuteflor groups, but there was a significant difference between probiotics-exposed and placebo groups. This might be due to the effect of probiotics on improving intestinal function and preventing the reabsorption of bilirubin in infants. These findings are similar to those in the systematic reviews by Deshmukh et al. (22), Ahmadipour et al. (23), and Torkaman et al. (24). However, in the study by Zahed Pasha et al. (16), no significant relationship was observed between probiotic consumption and phototherapy duration in term neonates. This disagreement could be attributed to the gestational age of infants and the different doses of the drug used. Furthermore, the comparison of the incidence of NEC, mortality, sepsis, and icterus showed no differences. Similar studies by Li et al. (25) and Escarate et al. (26) focused on NEC incidence, although several studies such as those by Nouri Shadkam et al. (11), Underwood (27), Bayani et al. (28), and Panchal et al. (29) indicated that probiotics reduce the incidence or severity of NEC and mortality. Moreover, Garg et al. (30) presented in their study that probiotics had a significant impact on the incidence of NEC, but were not effective in reducing NEC-related deaths. In our study, this result could be due to the very low incidence of NEC and mortality in newborns, possibly due to the low sample volume. There was no case of late-onset sepsis in the three groups.
5.1. Conclusions
The use of Reuteflor and Pedilact can be effective in reducing the number of days needed for phototherapy and oxygen therapy in premature infants, but its effect on RDS and BPD requires further study. Consumption of Pedilact (a synbiotic) was effective in increasing the weight gain of premature infants. The use of probiotics in this study did not affect the decrease in the incidence of icterus, NEC, and mortality.