1. Introduction
Calcifying pseudoneoplasm of the neuraxis (CAPNON) represents a rare, benign lesion of the central nervous system (CNS), first characterized by Rhodes and Davis in 1978 (1) With about 150 cases reported since its initial description, CAPNON remains an enigmatic entity, often misdiagnosed due to its rarity and nonspecific features (2, 3). These slow-growing, non-neoplastic lesions can occur anywhere along the neuraxis — including the brain, spinal cord, meninges, and adjacent tissues — exhibiting a slight predilection for intracranial sites (4, 5). The rarity of CAPNON, coupled with its variable presentation, poses significant diagnostic and management challenges, necessitating a comprehensive understanding of its clinical, radiological, and histopathological profiles as informed by prior studies. This introduction synthesizes the current knowledge of CAPNON, highlighting its significance within the broader field of CNS pathology and setting the stage for a novel case that contributes to this evolving landscape.
The clinical presentation of CAPNON is heterogeneous, driven primarily by the lesion’s location, size, and mass effect on surrounding structures. Common symptoms include headaches, seizures, and focal neurological deficits, though some lesions remain asymptomatic and are detected incidentally during imaging for unrelated conditions (6). Skull base CAPNONs, for instance, may manifest with cranial neuropathies due to compression of adjacent neurovascular structures, while spinal lesions often present with axial or radicular pain (4). This variability underscores the importance of correlating clinical findings with imaging and histopathological confirmation, as symptoms alone lack specificity for preoperative diagnosis.
Radiologically, CAPNONs are distinguished by their dense calcification, appearing hyperdense on computed tomography (CT) and typically hypointense on T1- and T2-weighted magnetic resonance imaging (MRI) sequences (4, 7). Enhancement patterns vary from minimal to heterogeneous or peripheral, with perilesional edema being an uncommon finding reported in select cases (8, 9). These features, while characteristic, overlap with other calcified CNS lesions — such as meningiomas, vascular malformations, and low-grade glial neoplasms — complicating differential diagnosis (5, 10). Consequently, imaging serves as a critical yet preliminary tool, with definitive diagnosis relying on histopathological evaluation.
Histopathologically, CAPNONs exhibit a distinctive profile, typically featuring a chondromyxoid matrix with calcifications, palisading spindle or epithelioid cells, and an inflammatory infiltrate (6, 11). Variability in these features across cases necessitates meticulous pathological examination to distinguish CAPNON from mimics like granulomatous diseases or neoplasms (3, 11). Immunohistochemical staining further aids diagnosis, with lesions commonly showing CD68 positivity in macrophages, variable vimentin and S100 expression, and negativity for glial fibrillary acidic protein (GFAP) and epithelial membrane antigen (EMA) in most instances (3). These findings support hypotheses of a reactive etiology, potentially linked to trauma, inflammation, or prior injury, though the precise pathogenesis remains elusive (5, 6).
Management of CAPNON centers on surgical resection, the primary treatment for symptomatic lesions, which is generally curative with a low recurrence rate (3, 12). Complete excision is preferred when feasible; however, in anatomically challenging locations such as the skull base, subtotal resection may be performed, carrying a small risk of recurrence (3, 13). To date, recurrence has been documented in only a handful of cases, predominantly following incomplete resection (14, 15). This favorable prognosis reinforces CAPNON’s benign nature, yet the rarity of recurrence and atypical presentations warrant continued investigation into optimal therapeutic strategies.
In this context, we report the case of a 54-year-old female with a left frontal lobe CAPNON presenting with urinary incontinence, bilateral arm weakness, and depressed mood. Notable for its atypical radiological feature of vasogenic edema and post-surgical regrowth requiring radiotherapy, this case highlights CAPNON’s potential for unexpected behavior and the need for tailored management approaches. By building on the foundation of prior works, this report aims to enhance the understanding of CAPNON’s clinical spectrum and therapeutic considerations.
2. Case Presentation
2.1. Presentation
A 54-year-old female patient presented to her primary care physician with complaints of urinary incontinence, bilateral arm weakness, and a depressed mood. Due to the neurological symptoms, a CT scan was performed to rule out an emergent intracranial hemorrhage, and the patient was then referred to a neurosurgeon. Subsequent MRI was ordered to further evaluate the findings.
2.2. Medical History
The patient had undergone a hysterectomy 8 months prior to the onset of neurological complaints. She also experienced a vertebral fracture due to a fall from standing height. Based on the MRI and surgery report, there were no signs of malignancy or neoplasms in the vertebra; only a fracture was present, which was subsequently repaired.
2.3. Diagnosis and Management
2.3.1. Initial Magnetic Resonance Imaging
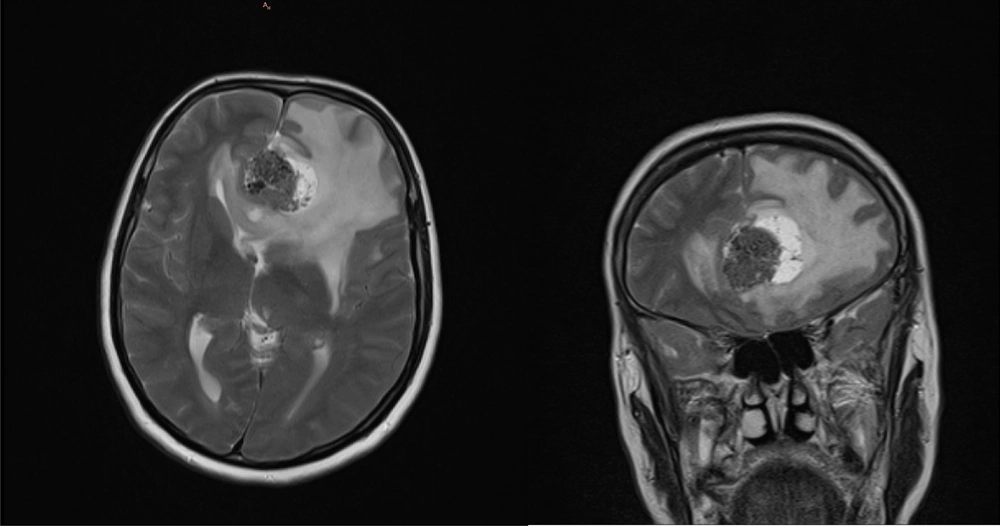
Initial MRI findings revealed a well-defined, extra-axial mass in the left frontal lobe with anterior falx attachment. The lesion measured 35 × 27 × 33 mm and had a mixed solid and cystic appearance. It appeared hypointense compared to gray matter on T1-weighted, T2-weighted, and fluid-attenuated inversion recovery (FLAIR) sequences. A central area of low signal intensity, consistent with calcification, was present within the mass. Surrounding vasogenic edema was observed in the adjacent brain parenchyma. The mass effect from the lesion caused compression of the frontal horn of both lateral ventricles. After intravenous contrast administration, the solid portion of the mass showed heterogeneous enhancement, while the cystic portion did not enhance. The initial MRI is presented in Figure 1.
2.3.2. Pathology
Following surgical resection, the pathology specimen consisted of multiple irregular, creamy-to-brownish tissue fragments with a soft consistency, measuring 3.5 × 3 × 1 cm in aggregate. Microscopic examination revealed brain tissue with multiple calcifications surrounded by mononuclear cells (macrophages) and lymphoplasmacytic cells. Immunohistochemical staining was positive for CD68 in the mononuclear cells and negative for cytokeratin (CK). The examination also showed multiple rounded foci with peripherally calcified centers composed of histiocyte aggregates and a lymphoplasmacytic infiltrate. Fibrosis and reactive surrounding brain tissue were present. No evidence of a neoplastic process was identified. The final pathological diagnosis was CAPNON.
2.3.3. Post-surgical Recovery
Immediately following the surgery, the patient began to feel better. The weakness in her arms and urinary incontinence diminished, and her mood improved. These improvements were noted during the initial postoperative assessment, indicating a positive response to the surgical intervention.
2.3.4. 3-Month Follow-up Magnetic Resonance Imaging
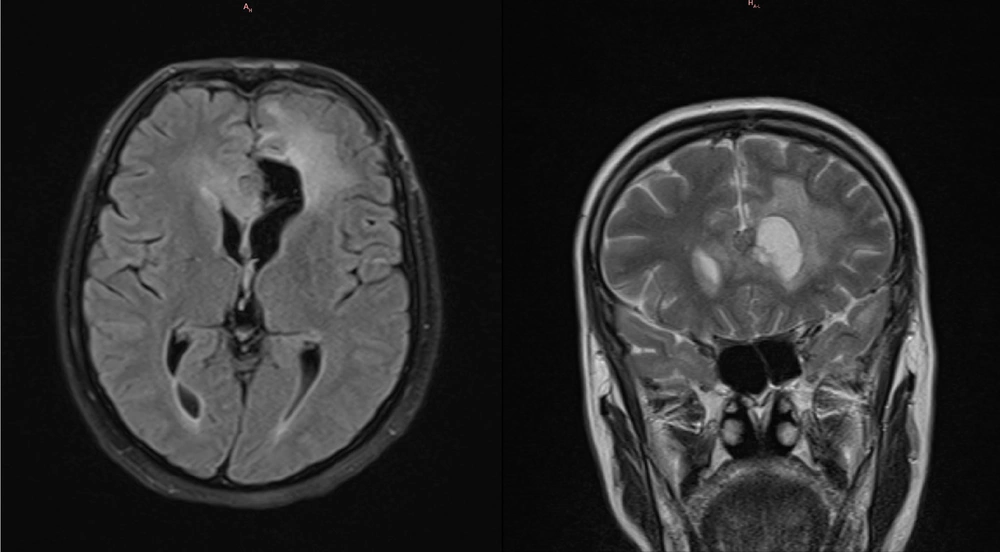
The first follow-up MRI without contrast was performed at approximately 3 months as part of the standard postoperative procedure. Only cystic components were visible in all views, except in the coronal view, where a suspicious residual solid tumor was noted. It must be noted that since this MRI did not include contrast dye, the interpretation is not definitive. The imaging showed a decrease in the surrounding edema. The patient reported no new or worsening symptoms at any point following the surgery. The imaging findings are presented in Figure 2.
2.3.5. 8-Month Follow-up Magnetic Resonance Imaging
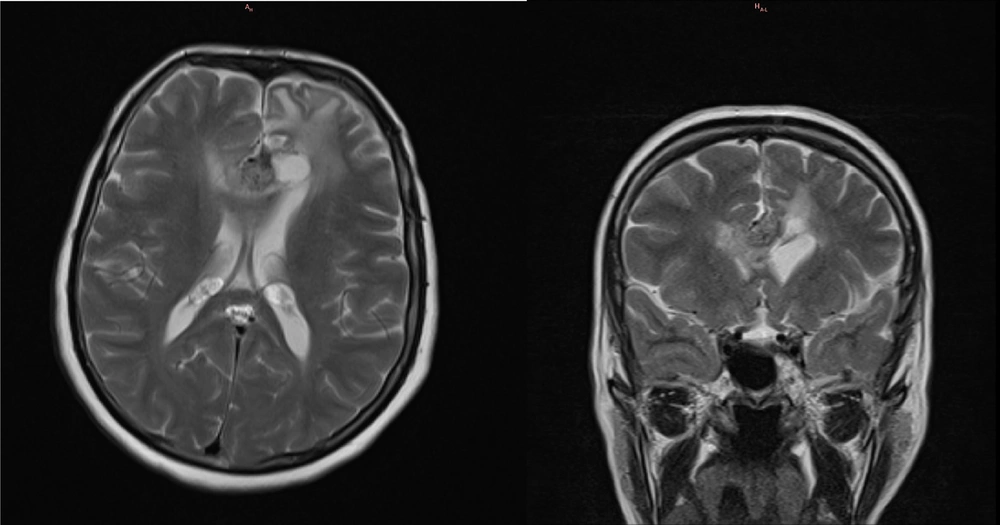
The second follow-up MRI with contrast was performed at approximately 8 months as part of the standard postoperative procedure. The imaging showed a drastic decrease in the surrounding edema; however, an increase in the size of the residual solid component was observed, with imaging characteristics similar to the original tumor, measuring 18 × 15 × 13 mm. Despite this, the patient reported no new or worsening symptoms at any point following the surgery. The imaging findings are presented in Figure 3.
2.3.6. 12-Month Follow-up Magnetic Resonance Imaging
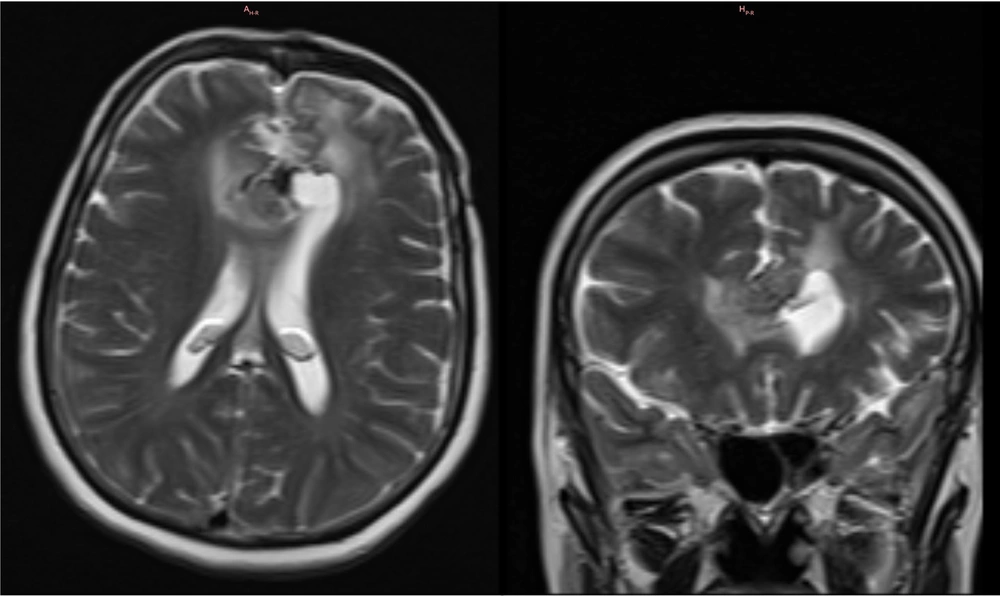
A subsequent third follow-up MRI with contrast was conducted at approximately 12 months, also as part of the standard postoperative procedure. The imaging revealed an increase in the size of the residual tumor to 21 × 24 × 26 mm, with no change in its signal characteristics, persistent edema, and no hydrocephalus. Despite this radiological progression, the patient reported no new or worsening symptoms at any point after the surgery. The final imaging findings are presented in Figure 4.
2.3.7. Radiotherapy
Due to the documented tumor regrowth and following review at the multidisciplinary tumor board, the patient was referred for adjuvant radiotherapy. The chosen treatment was intensity-modulated radiation therapy (IMRT), which delivered a total dose of 54 Gy in 27 fractions.
3. Discussion
Calcifying pseudoneoplasm of the neuraxis is a rare, benign CNS lesion that remains poorly understood due to its rarity and variable presentation. Since its initial description by Rhodes and Davis in 1978 (1), fewer than 150 cases have been documented, with most occurring intracranially and presenting with symptoms such as headaches, seizures, or focal neurological deficits driven by mass effect (2, 3). Radiologically, CAPNONs are characterized by dense calcification on CT and hypointensity on T1- and T2-weighted MRI sequences, often with minimal enhancement (4, 7). However, their nonspecific imaging features frequently lead to diagnostic confusion with entities like meningiomas, vascular malformations, or low-grade glial neoplasms (5, 10). Histopathologically, CAPNONs exhibit a distinctive chondromyxoid matrix with calcifications, palisading spindle or epithelioid cells, and an inflammatory infiltrate, supporting a possible reactive etiology (6, 11).
This case report of a 54-year-old female with a left frontal lobe CAPNON highlights several unique features that distinguish it from prior reports, including the presence of vasogenic edema, post-surgical tumor regrowth necessitating radiotherapy, and a potential link to prior trauma or surgery. In this case, the atypical finding of vasogenic edema is a significant departure from the typical radiological profile of CAPNONs, where perilesional edema is rare (3, 4). In the literature, vasogenic edema has been documented in only a handful of cases, often correlating with symptomatic progression or larger lesions exerting greater mass effect (8, 9). Here, the initial MRI revealed a 35 × 27 × 33 mm extra-axial mass with mixed solid and cystic components, central calcification, and surrounding edema compressing the frontal horns of the lateral ventricles. This edema likely contributed to the patient’s presenting symptoms of urinary incontinence, bilateral arm weakness, and depressed mood — symptoms less commonly associated with CAPNONs compared to headaches or seizures (12). The frontal lobe location may explain the mood alterations, given its role in emotional regulation, and the resolution of symptoms post-surgery suggests that mass effect and edema were primary drivers of the clinical picture rather than intrinsic lesion behavior.
In this case, the histopathological findings align with CAPNON’s classic features — calcifications within a chondromyxoid matrix, surrounded by inflammatory cells staining positive for CD68 — but also prompt speculation about its pathogenesis. The patient’s history of a vertebral fracture and hysterectomy suggests a potential association with prior trauma or surgery, supporting theories that CAPNON may represent a reactive process to injury or inflammation (5, 6). Although no malignancy was identified in the vertebral fracture, the temporal proximity of these events to the neurological presentation raises the possibility of a systemic predisposition to aberrant healing responses. Furthermore, the mixed solid-cystic nature of the lesion, with a non-enhancing cystic component, is infrequently reported and may reflect trapped cerebrospinal fluid or a localized inflammatory reaction contributing to regrowth (16). Unlike cases with collision lesions (e.g., meningiomas or lipomas), no coexisting pathology was identified here, making the cystic feature an intrinsic characteristic of this CAPNON (6).
Surgical resection is widely regarded as curative for CAPNONs, with recurrence reported in only six cases (3, 14, 15). In this patient, subtotal resection resulted in a residual solid component evident on the 8-month follow-up MRI (18 × 15 × 13 mm), which unexpectedly grew to 21 × 24 × 26 mm by 12 months, despite the absence of symptom recurrence. This regrowth challenges the conventional view of CAPNON as a uniformly indolent entity. It contrasts with prior reports where rare recurrence was typically linked to incomplete resection at surgically challenging sites, such as the skull base (13). The decision to pursue intensity-modulated radiotherapy (IMRT) at 54 Gy in 27 fractions marks a novel therapeutic approach for CAPNON, as the literature seldom describes adjuvant therapies. While Lu et al. suggest serial imaging for monitoring residual skull base lesions, the accessible frontal lobe location, in this case, raises questions about why regrowth occurred. Possible explanations include microscopic residual disease not addressed by surgery or an unusually active inflammatory process driving proliferation, as evidenced by the histopathology showing mononuclear and lymphoplasmacytic infiltrates (6).
In this case, radiotherapy differentiates it from prior discussions, where management has focused almost exclusively on surgical resection (3, 13). The literature offers little guidance on adjuvant therapies, with most reports emphasizing the benign nature of CAPNON and the efficacy of complete excision (12). Traditionally, the absence of radiotherapy in CAPNON management protocols stems from its benign histopathology, slow growth kinetics, and the high rates of curative resection. Recent institutional series and meta-analyses have also shown that even incomplete resections rarely require postoperative radiation, as recurrence rates remain low with residual disease (17). However, our case developed recurrence, prompting the use of adjuvant IMRT. This patient’s asymptomatic radiological progression necessitated an alternative strategy, and IMRT was selected to target the growing residual mass. For benign or low-grade CNS tumors, moderate-dose radiotherapy (45 – 54 Gy) is typically employed to balance tumor control with toxicity risks. In meningiomas that are grade 1, for example, doses of 50 – 54 Gy have been shown to achieve 5-year local control rates exceeding 90% (18). Similarly, studies in craniopharyngioma report that 54 Gy is effective, with 10-year overall survival and local control rates of 86.4% and 92.7%, respectively (19). These findings support the use of 54 Gy for slow-growing lesions such as CAPNON. This approach aligns with managing other benign but recurrent CNS lesions, yet its application in CAPNON is unprecedented and warrants further investigation.
The patient’s sustained symptomatic improvement despite tumor growth suggests that clinical outcomes may not always correlate with radiological findings, a phenomenon possibly explained by the initial relief of mass effect and edema rather than eradicating the lesion itself. The long-term outcomes and consequences of the IMRT administered in this case will be the subject of a future editorial letter.
Diagnostically, this case underscores the challenge of preoperative identification of CAPNON. The initial radiological impression likely favored a meningioma due to the extra-axial location, dural attachment, and calcification — features overlapping with CAPNON (7). However, vasogenic edema and a cystic component diverge from the typical meningioma profile, which more commonly exhibits homogeneous enhancement and less pronounced edema unless atypical or large (5). This diagnostic ambiguity reinforces the critical role of histopathology, as imaging alone cannot reliably distinguish CAPNON from its mimics, including oligodendrogliomas, cavernomas, or granulomatous processes (3, 10). Clinicians should thus include CAPNON in the differential diagnosis of calcified brain lesions, particularly when atypical features are present.
3.1. Conclusions
In conclusion, this case of a frontal lobe CAPNON with vasogenic edema, post-surgical regrowth, and subsequent radiotherapy offers a distinct perspective from previous reports. It highlights the heterogeneity of CAPNON’s clinical behavior and challenges the assumption of uniform benignity and surgical curability. The potential link to prior trauma, inflammatory histopathology, and the novel use of radiotherapy suggests avenues for future research into CAPNON’s pathogenesis and management. As a rare entity, CAPNON requires heightened diagnostic suspicion, histopathological confirmation, and, in select cases, individualized treatment beyond surgery to address unexpected progression.