1. Background
Wound is a breakage in the continuity of body tissue due to violence (1). The violence would be caused by many conditions such as trauma, surgery, burn (2), or diabetic and pressure ulcers (3). Wounds, especially chronic wounds, affect 6.5 million patients in US and cost an estimated US $25 billion annually (4). The number of chronic wounds is growing worldwide due to increased age-related conditions and disorders such as obesity, diabetes, and heart failure diseases. Moreover, the burden is expected to grow more due to the rise of healthcare costs, an aging population, and a global rise in the occurrence of diabetes and obesity (5-7). Current therapeutic agents used for wound healing are inefficient due to their large number of serious adverse effects (4). Nowadays, the global interest in use of natural products and herbal therapy are increasing for their high acceptability and good toleration. The medicinal plants are well-known for their efficiency to promote wound healing and preclude infection without prominent side effects (8-10).
Wound healing is a progression of biophysiological events as a response to tissue damage, which includes the interaction of cellular components, growth factor, and cytokines. It is divided into 4 sequential and overlapping phases of hemostasis, inflammation, proliferation or maturation, and remodeling (11). All phases arise in a proper sequence and time frame (12). The purpose of wound healing is to shorten its time frame and decrease its side effects in order to improve the quality of life (13).
Angiogenesis is an advanced feature, which characterizes the proliferative phase of healing (14, 15). Angiogenesis is a process of forming new blood vessels from pre-existing vessels adjacent to the wound. In response to injury, an angiogenetic process begins by the invasion of microvascular endothelial cells including activation of endothelial cells, degradation of their basement membrane, sprout development into the wound clot, cell proliferation, capillary tube formation, recreation of basement membrane, and eventually regression of newly formed vessels as tissue remodeling (16). Granulation tissue formation is an essential factor to assay the quality of wound healing. The granulation tissue formation is actually due to the accumulation of fibroblast cells, increased blood vessels, and synthesis of collagen fibers (17). VEGF (vascular endothelial growth factor) is a homodimer glycoprotein that initiates the formation of granulation tissue and angiogenesis (9, 18). In fact, extension of angiogenesis directly depends on expression of this factor.
Resveratrol (3, 5, 4’-trihydroxystilbene) is a natural polyphenol found mainly in red grapes and mulberries. Many studies demonstrated its antioxidant (19), anti-inflammatory (20), anti-aging, and chemo preventive functions (21). Resveratrol can also protect the blood vessels from atherosclerosis (22). In addition to its beneficial pharmacological effects, the topical application of resveratrol has therapeutic action opposed to cutaneous pathologies such as oxidative damage (23, 24). Due to the poor bioavailability of resveratrol resulted from its rapid metabolism and limited solubility in water, topical administration of resveratrol can circumvent this problem (24). This polyphenol has the ability to regulate the VEGF expression and promote angiogenesis (25).
There are variable and complex reports of resveratrol on angiogenesis. As it already mentioned, this polyphenol has proangiogenic effects on the ischemic myocardium, but there is evidence based on anti-angiogenetic function of this polyphenol in several types of cancers (26). For these conflicts the current study aimed at clarifying the role of resveratrol on angiogenesis during the wound healing process in mice.
2. Methods
2.1. Animals
This experimental study was performed on 2.5-month-old male BALB/c mice (n = 60) weighting 25 ± 5 g supplied by the research center of experimental medicine at Birjand University of Medical Sciences, Birjand, Iran. The mice were housed in individual cages (12:12 hour light/dark cycle at 22 - 24°C) and were fed standard food and water ad libitum. The experimental procedures used in the current study were approved by the ethic committee of laboratory animals at Birjand University of Medical Sciences (ir.bums.REC.1395.293), Birjand, Iran.
2.2. Incisional Dermal Wound Technique
Mice were anesthetized following ketamine hydrochloride (70 mg/kg) and xylazine (13.4 mg/kg) injection intraperitoneally (IP). Then, after shaving an incisional wound (2 cm) with the depth of full-dermis thickness incision was placed on the dorsal skin (paravertebral), equidistant from the midline in sterile conditions. Digital imaging of wound was performed utilizing a digital camera (Mavica FD91, Sony). Then, wounds of each group were topically treated with creams, as already explained, twice a day with 12 hours interval between each application for 14 consecutive days from the day of wounding (the day 0). Three mice of each group were sacrificed using ether after 4, 7, 10, and 14 days post-wounding and 1 - 1.5 mm of wound edges were excised for histological and immunohistochemical studies (18).
Mice were randomly divided into 5 groups (each group n = 12). The intervention groups were treated with 0.15% and 0.05% resveratrol cream, respectively. The negative control group received eucerin plus polyethylene glycol (PEG)-400. The positive control group received nitrofurazone 0.2% and the sham group received no treatment (normal healing).
2.3. Reagents
Resveratrol (> 99% pure, from Sigma) was purchased from Steinheim, Germany.
The excipients for the cream were provided by Behvazan Pharmaceutical Co. Tehran, Iran.
2.4. Cream Procedure Consumption
Creams were prepared daily and kept in a dark container. To provide 1% and 0.05% resveratrol creams, respectively 100 and 50 mg resveratrol were dissolved in PEG-400 with emulsifying wax (eucerin). The control group was added to the study, receiving the cream containing PEG-400 plus eucerin and 2% nitrofurazone, due to better analysis and comparison of angiogenetic effect of resveratrol on healing the wound.
2.5. Histology
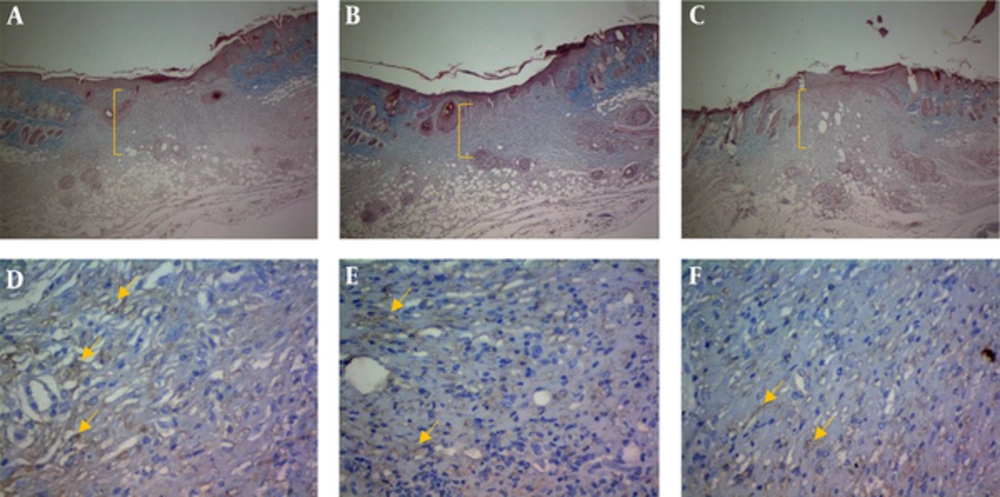
Formalin fixed wound edges were paraffin-embedded and sectioned. The 5-μm sections were deparaffinized and stained using hematoxylin and eosin and Masson trichrome techniques to assess morphological details and compare with the immunostained sections (18). Masson trichrome staining technique results in blue-black nuclei, blue cytoplasm and collagen, and red muscle and keratin (18).
2.6. Immunohistochemistry
Endothelial cells were highlighted in tissue samples by immunostaining for VEGF antigen. Five-micrometer sections were placed on charged slides and rehydrated. All washings were done at room temperature. Sections were washed 3 times in 0.05 M Tris-buffered saline (Dako TBS, code S3001) (pH 7.6) and treated with retrieval solution (pH 9). Endogenous peroxidase activity was blocked with 0.3% (v/v) H2O2 in TBS. The slides were washed 3 times in TBS. Next, they were incubated for 30 minutes with anti-VEGF rabbit polyclonal antibody (ready-to-use (RTU) (BioGenex, code: AR483-5R, United States) at 40°C. Next, the slides were washed with TBS and incubated with streptavidin-horseradish peroxidase (HRP) (Dako, code P0260) secondary antibody for 20 minutes at 40°C. It was followed by washing the slides with TBS and incubating with substrate-chromogen solution (3,3-diaminobenzidine, DAB from Dako) for 10 minutes and counterstained with Mayer hematoxylin for 10 seconds. The slides were then dehydrated and mounted. Images were obtained using an Olympus SZX microscope fitted with Pixera digital camera and software (18).
2.7. Vascular Density Determination
Granulation tissue vessel density and appearance of neovascularization at different stages of wound healing was measured with ImageJ software (18).
2.8. Statistical Analysis
Data were expressed as mean ± SD. Intergroup comparisons were performed by analysis of variance (ANOVA). Data obtained from wounds in the intervention groups were compared with those of the control groups using Tukey test. P < 0.05 was considered statistically significant. Data were analyzed with SPSS version 24.
3. Results
All mice survived during surgical procedure and there was no death of mice during the study. The mean values of wound angiogenesis and granulation tissue on the post-operative days (PODs) 7, 10, and 14 of the intervention and control groups and the statistical comparisons of the groups are shown in Table 1.
| Day | Granulation, μm2 | VEGF-Expression (OD) |
|---|---|---|
| 7 | ||
| R 0.1 | 1258460.04 ± 74325.0c,d | 0.127 ± 0.01c,d |
| R 0.05 | 1176000.93 ± 53890.87c,d | 0.102 ± 0.014c,d |
| Nit 0.2% | 1020313.79 ± 105540.33 | 0.083 ± 0.009 |
| Eucerine, PEG-400 | 955840.89 ± 46093.98 | 0.077 ± 0.014 |
| None | 837044.56 ± 87786.05 | 0.069 ± 0.001 |
| 10 | ||
| R 0.1 | 2055932.27 ± 180124.66c,d | 0.092 ± 0.008c,d |
| R 0.05 | 2029197.75 ± 143942.96c,d | 0.083 ± 0.003c,d |
| Nit 0.2% | 1657661.86 ± 78112.47c | 0.076 ± 0.002c |
| Eucerine, PEG-400 | 1463358.30 ± 99134.63 | 0.068 ± 0.003 |
| None | 1326564.45 ± 94557.34 | 0.063 ± 0.002 |
| 14 | ||
| R 0.1 | 1807574.38 ± 52802.98c,d | 0.085 ± 0.007c,d |
| R 0.05 | 1719318.03 ± 62480.33c,d | 0.080 ± 0.002c,d |
| Nit 0.2% | 1599610.35 ± 51559.68 | 0.070 ± 0.001c |
| Eucerine, PEG-400 | 1539553.47 ± 52358.08 | 0.067 ± 0.001 |
| None | 1464849.43 ± 42209.30 | 0.056 ± 0.003 |
aGroups: R 0.1, Resveratrol 0.1% Treated Wound; R 0.05, Resveratrol 0.05% Treated Wound; Nit, Nitrofurazone Treated Wound; Euc, Eucerine Treated Wound; None: Sham Group.
bData are expressed as mean ± SD.
cP < 0.05: compared with the control and sham groups.
dP < 0.05: compared with the nitrofurazone group (OD: optical density).
During the wound healing process, fibroblast cells proliferation, granulation, angiogenesis, and synthesis of collagen fibers occurred after the day 6; therefore, there was no evidence of such parameters except inflammatory cells on the day 4 (data not shown), and these parameters were not detectable.
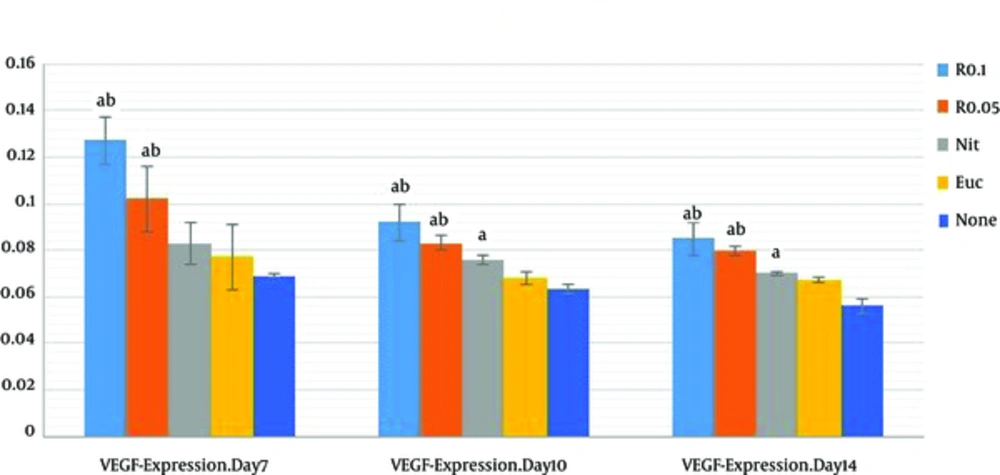
The results of VEGF-Immunostaining showed that the VEGF-presence mean value of resveratrol-treatment groups (R 0.05, R 0.1) were markedly higher than those of control and sham groups on PODs 7, 10 and 14 (P < 0.001); resveratrol-treatment groups were associated with higher presence of VEGF in a nearly concentration-dependent manner at wound site. There was an increase of VEGF presence in 0.1% resveratrol-treated group (R = 0.1) compared with 0.05% resveratrol-treated group (R 0.05) on POD 7, 10, and 14 (P < 0.001 each). And no significant difference was observed between R 0.1 and R 0.05 on PODs 10 and 14 (P = 0.10 and P = 0.68, respectively) (Table 1).
The mean values of VEGF presence in R 0.1 and R 0.05 significantly increased compared with those of nitrofurazone group on PODs 7, 10, and 14 (P < 0.05).
As shown in Figure 1, VEGF on the POD7 was at highest comparing with other days. Results of the current study showed that VEGF level decreased on the PODs 10 and 14. It can be explained that the regression of many of the newly formed capillaries occur after the POD 7.
Data are expressed as mean ± SD, n = 3 in each group, R 0.1, resveratrol 0.1% treated wound; R 0.05, resveratrol 0.05% treated group; Nit, nitrofurazone treated group; Euc, eucerine treated group; None, sham group. One-way ANOVA followed Tukey’s post-hoc test. aP < 0.05: compared with the control and sham groups, bP < 0.05: compared with the nitrofurazone group (OD: optical density).
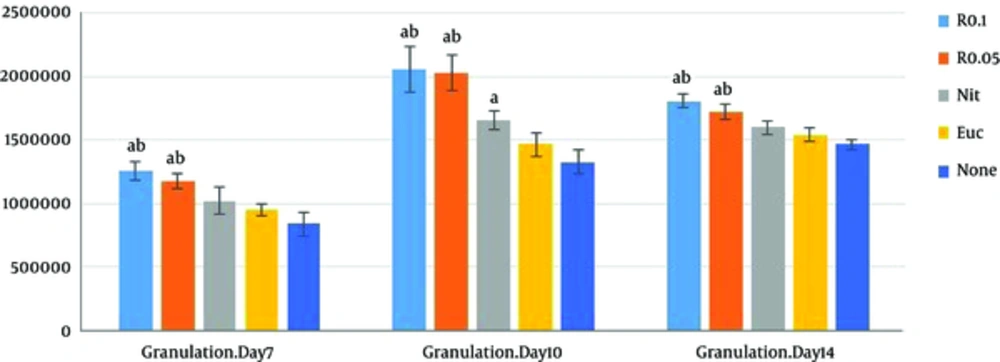
Another finding of the current study was related to the role of resveratrol in wound granulation tissue. Verification of the role of resveratrol in wound granulation tissue is another finding of the present study (Figure 2). The trichrome staining of wound tissue showed that the granulation mean values of resveratrol 0.1% and 0.05% intervention groups (R 0.05, R 0.1) were markedly higher than those of the control and sham groups on the PODs 7, 10, and 14 (P < 0.001 each); resveratrol treated groups were associated with increased epidermis formation (data not shown) and deposition of connective tissue compared with those of the control and sham groups. There was a significant increase in granulation extension in R 0.1 compared with that of R 0.05 on the POD 14 (P = 0.004), but no significant difference was observed between R 0.1 and R 0.05 on the PODs 7 and 10 (P = 0.13, P = 0.98 respectively) (Table 1).The wound tissue of eucerine-treated and sham groups indicated less extensive granulation tissue and below epidermis formation (Figure 3). There was a significant difference between nitrofurazone, and control and sham groups on the POD 10 (P < 0.001), but no significant difference was observed between nitruforazone, and control and sham groups on the POD 7 (P = 0.34) and the POD 14 (P = 0.09) (Table 1).
Values are expressed as mean ± SD, n = 3 in each group R 0.1, resvertrol 0.1% treated wound; R 0.05, resveratol 0.05% treated group; Nit, nitrofurazone treated group; Euc, eucerine treated group; None, sham group. One-way ANOVA followed by Turkey’s post-hoc test. aP < 0.05: compared with the control and sham groups, bP < 0.05: compared with the nitrofurazone group.
4. Discussion
Wound healing is a complex process of 4 stages including: hemostasis, inflammation, proliferation, and remodeling. In proliferative phase, angiogenesis is critical and (14) VEGF also has powerful and long-time effects on angiogenesis in wounds. It prompts migration and proliferation of endothelial cells and upgrades blood vessels permeability (27). Resveratrol is a naturally polyphenolic compound derived from grapes (28).
The current study hypothesized that resveratrol may end in improving wound healing. To the authors ‘best knowledge, topical application of resveratrol at a dose-dependent manner may result in great amount of VEGF expression and increased blood vessels at newly formed granulation tissue. This can lead to greater blood flow to the wound, which causes elevated perfusion of healing factors and it consequently facilitates the healing process.
To prove whether resveratrol may increase angiogenesis, the VEGF presence was assayed at wound granulation tissue by immunostaining. Resveratrol-treatment was associated with markedly higher presence of VEGF in a nearly concentration-dependent manner (100 mg) at wound site. Based on the idea that angiogenesis is a specialized feature of proliferative phase of healing, great amount of VEGF expression was observed on the POD 7. The increasing angiogenesis results in richer blood supply to the wound, which causes increased perfusion of healing factors and facilitates the healing process. The VEGF decreased on the PODs 10 and 14 compared with the POD 7. It can be explained that the regression of many of the newly formed capillaries occur on such days and the vascular density of the wound approaches to normal.
Some studies show variable results of resveratrol in angiogenesis. This polyphenolic compound increases endothelial NO synthase (eNOs) activity and stimulates VEGF expression (14, 27, 29, 30). It is also reported that natural extracts derived from grape seeds facilitate VEGF expression induced by oxidant in keratinocytes (27). In a murine model of excisional wound in dermis, grape seed extract in combination with 5000 ppm resveratrol remarkably accelerated wound contraction and healing (29). Grape seed extract containing resveratrol manages transcriptional control of inducible VEGF expression by regulating pathways that are common on both H2O2 and TNFα signaling (27). It is also reported that resveratrol notably develops myocardial angiogenesis in a rat myocardial infarction (MI) model through a thioredoxin-, NO-, heme oxygenase- and VEGF-related method (31).
On the contrary, some studies showed anti-angiogenetic function of resveratrol to inhibit tumor growth mediated through the suppression of blood vessel growth. The combination of resveratrol (10 - 100 µg/mL) and quercetin affected the apoptosis and proliferation of human tumor and endothelial cells in a dose dependent manner and was suggested as an antitumor drug (32). Lower tumor growth was also observed due to decreased angiogenesis via reduced extracellular levels of VEGF and increased apoptotic index in ERα-ERβ+ MDA-MB-231 tumors in resveratrol treated MDA-MB-231 cells in breast cancer (at a dose of 25 mg/kg/day) (33).
The biphasic angiogenetic effect of resveratrol was proved by chick chorioallantoic membrane assessment and it was observed that both AKt and ERK signaling pathways, functional for angiogenesis, become stimulated in response to 5 µM of resveratrol (25). While resveratrol at low concentrations, from 1 to 10 µM, upregulates the expression of VEGF and promotes angiogenesis, it has opposite effects at high concentrations (20 µM and higher) on angiogenesis (25).
Since granulation tissue is needed to reconstruct dermal integrity, and it is also required for crucial re-epithelialization, it is essential to assay granulation tissue formation to find the quality of wound healing. Furthermore, granulation tissue is vital for skin graft in surgical operations. It is reported that VEGF initiates the formation of granulation tissue. The formation of granulation tissue is actually due to the accumulation of fibroblast cell, increase in the number of blood vessels, and synthesis of collagen fibers (17).
In the current study, the effectiveness of resveratrol on granulation tissue extension was investigated. The results showed that resveratrol improved granulation tissue formation and increased density of granulation tissue by enhancing angiogenesis.
A study by Yaman et al., on skin wound healing in rats showed that oral administration of resveratrol affected fibroblast proliferation, increased the synthesis of collagen fibers, which causes increased granulation extension and improved wound healing (14). Furthermore, a lot of researches are focused on anti-inflammatory properties of resveratrol and its analogs by modulating secretion of several cytokines and chemokines (20, 34, 35). The inflammatory cells produce these mediators immediately after injury to initiate the proliferative phase of wound healing. The anti-inflammatory effect of resveratrol by blocking nuclear factor-κB (NF-kB) signaling pathway is emphasized in some studies (36). All of these reports show that resveratrol may accelerate passing the inflammatory phase to the next proliferation phase by modulating secretion of cytokines and chemokines and shortening the healing proper time frame.
In conclusion, the current study findings confirmed that resveratrol had a positive effect on incisional wound healing. The results of the current study demonstrated that it could confer profits on tissue healing by significant increase of VEGF expression and extensive granulation tissue formation on wound healing process in Balb/c mice. Further clinical investigations are needed to clarify the benefits of resveratrol to promote wound healing.