1. Background
Asthma is globally the most prevalent chronic condition of childhood and adolescence (1, 2) It has an increasing prevalence (3) so that the number of afflicted patients worldwide is estimated to reach 400 million by 2020 (4). Asthma is a chronic inflammatory disease of the airways that results in the oversensitivity of the airways, mucosal edema, and excessive mucus production. It causes recurrent symptoms such as a cough, lung tightness, wheezing, and dyspnea, all of which are very discomforting for patients and can negatively affect their daily functioning and quality of life (5).
Disease control is the main goal of long-term management of asthma. The term “asthma control” means the extent to which an afflicted patient can reduce or even eradicate the clinical manifestations of the disease. Asthma control has two main components. The first is the clinical control that includes the management of current symptoms and the improvement of patient’s ability to do activities of daily living in order to achieve the highest possible level of quality of life. The other component of asthma control is the control of complications and the prevention of disease aggravation (6). However, most patients may be unable to effectively control their asthma even with quality medical treatments (7) and hence, psychological interventions may be needed to help improve asthma control.
Motivational interviewing (MI) is among the newest methods for behavior modification (8). It was developed by Miller and Rollnick (9) based on Rogers’ client-centered therapy approach to psychotherapy (10). The MI is an attempt for promoting individual’s self-efficacy and self-control for behavior modification. The MI works based on motivation enhancement through interactive and sympathetic listening. It greatly focuses on the difference between personal goals and current behaviors (11). The MI enables clients to explicitly articulate their ambivalences, identify their conflicting motivations and satisfactorily solve them, and modify their behaviors under the direct guidance and supervision of an interviewer (9). It is a flexible method and can be used in either personal or group interviews. The latter provides the hope for successful problem solving, facilitates information acquisition from reliable sources, reduces social isolation, promotes altruism, and facilitates cognitive behavior modifications (12).
A study on 25 adults with asthma reported that MI significantly promoted their medication adherence (13). Another study also reported its effectiveness in significantly promoting self-efficacy among 37 adolescents with asthma (14). Other studies also reported the positive effects of MI on the different aspects of behavior among patients with chronic conditions such as renal failure and hemodialysis (15), psoriasis (16), and hypertension (17). However, the above-mentioned study on 37 adolescents with asthma reported the insignificant effects of MI on medication adherence (14). Moreover, most previous studies on asthma control used educational interventions rather than MI. For instance, an earlier study reported the positive effects of asthma education on asthma control (7).
2. Objectives
Due to the lack of studies into the effects of MI on asthma control, the present study was carried out to evaluate the effects of MI on asthma control among adolescents with asthma.
3. Methods
This randomized controlled clinical trial was conducted in 2017 on 64 adolescents with asthma who referred to the clinic and the Pediatric Ward of Valiasr (PBUH) Hospital, Birjand, Iran. The sample size was calculated based on the results of a former study (8) and with a µ1 of 19.7, a µ2 of 14.2, an S1 of 3.6, an S2 of 5.6, a power of 0.90, and a confidence level of 95%. The sample size calculation (Equation 1) showed that 29 participants were needed for each group. The sample size was increased to 32 considering an attrition rate of 10%.
Equation 1. Sample size calculation.
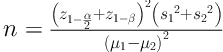
A convenience sampling was used to recruit eligible adolescents to the study. The eligibility criteria were an age of 10 - 15, asthma diagnosis by a specialist at least one year before the study, willingness for participation in the study, parental consent for participation, basic literacy skills, use of at least one inhaled asthma medication, and no mental disorders. The exclusion criteria included more than one absence from MI sessions and withdrawal from the study due to events such as accident or death. At the first face-to-face contact with participants and their parents, we informed them about the study and obtained parents’ consent for participation. For random allocation of participants to either of the study groups, each of them was provided with a card labeled A (i.e. control group) and a card labeled B (i.e. intervention group) and was asked to randomly select one card. The label on the card determined the group allocation of the intended participant.
Two questionnaires were used for data collection. The first was a demographic questionnaire with items on age, gender, education level, family income, father’s and mother’s employment status, and place of residence. The second was the Asthma Control Questionnaire with seven items on nighttime awakening, symptom severity upon awakening, activity limitation, shortness of breath, wheezing, number of sprayed puffs, and forced expiratory volume in the past week. Item scoring was done using a seven-point scale from zero (“no symptom or limitation”) to six (“severe symptom or impairment”). The total score of the questionnaire ranged from zero (good asthma control) to 42 (poor asthma control). A former study in Iran reported a Cronbach’s alpha of 0.80 for the Persian version of the questionnaire (18).
Before the intervention, all participants filled out the study questionnaires. Then, participants in the intervention group were divided into two eleven-person and one ten-person subgroups and then, each subgroup was provided with MI in five 60 - 80-minute weekly sessions. The MI sessions were held based on the approach recommended by Miller and Rollnick (19). Each participant was required to attend each MI session with either of his/her parents. In the first session, the participants and the interviewer (i.e. the first author) got familiar with each other and group norms and regulations were explained. Then, group members were evaluated respecting their latitude, independence, commitment, confidence, and factors affecting their behaviors. The second session was on the assessment of participants’ feelings about their asthma-related behaviors. Accordingly, a list of positive and negative feelings was provided to each participant and he/she was asked to identify his/her feelings and explain them for other group members. In the third session, brainstorming was used to assess the short- and long-term advantages and disadvantages of behaviors. The positive and negative aspects of each behavior were written in a table and behavior modification strategies were described and exercised. In the fourth session, participants’ values were identified and prioritized, value-behavior gaps were identified, and strategies for filling the gaps were exercised. Finally, in the fifth session, tempting situations were identified and the content of previous sessions was reviewed. On the other hand, participants in the control group received no specific intervention. The posttest was performed for all participants one week and three months after the final MI session.
Study data were analyzed via the SPSS software (V. 19.0). First, the groups were compared concerning participants’ demographic characteristics through the chi-square test. Then, the Kolmogorov-Smirnov test was performed to test the normality of asthma control scores. Its results revealed the normal distribution of the scores. Subsequently, the repeated-measures analysis of variance, the independent-sample t, and Bonferroni’s post hoc tests were used to analyze the data at a significance level of less than 0.05.
3.1. Ethical Considerations
The ethical approval and necessary permissions for the study were obtained from Birjand University of Medical Sciences, Birjand, Iran (approval code: IR.BUMS.REC.1395.179). Moreover, arrangements for the study were made with the authorities of the study setting. Participants and their parents were provided with information about the study and the confidential management of the study data. Then, parent informed consent was secured.
4. Results
The mean of participants’ age was 11.66 ± 1.62. The mean age in the control and intervention groups was 11.96 ± 1.84 and 11.56 ± 1.41, respectively. Most participants in both groups were males (62.5% and 59.4%, respectively) and all of them were students. Study groups did not significantly differ from each other concerning participants’ age, gender, education level, father’s and mother’s employment status, family income, and place of residence (P > 0.05; Table 1).
| Characteristics | Intervention (n = 32) | Control (n = 32) | P Valueb |
|---|---|---|---|
| Gender | 0.79 | ||
| Female | 13 (40.6) | 12 (37.5) | |
| Male | 19 (59.4) | 20 (62.5) | |
| Education level | 0.22 | ||
| Primary | 23 (71.9) | 21 (65.6) | |
| Guidance school | 2 (6.2) | 0 (0.0) | |
| High school | 7 (21.9) | 11 (34.4) | |
| Father’s employment | 0.82 | ||
| Self-employed | 23 (71.9) | 21 (65.6) | |
| Employee | 6 (18.8) | 8 (25.0) | |
| Teacher | 3 (9.3) | 3 (9.4) | |
| Mother’s employment | 0.79 | ||
| Employed | 21 (65.6) | 22 (68.7) | |
| Housewife | 11 (34.4) | 10 (31.3) | |
| Place of residence | 0.08 | ||
| Urban areas | 31 (96.9) | 27 (84.4) | |
| Rural areas | 1 (3.1) | 5 (15.6) | |
| Family income ($) | 0.75 | ||
| Less than 85 | 9 (28.1) | 11 (34.4) | |
| 86 - 210 | 21 (65.6) | 20 (62.5) | |
| More than 210 | 2 (6.3) | 1 (3.1) |
a Values are expressed as No. (%).
b The results of the chi-square test.
The between-group difference respecting the pretest mean score of asthma control was not statistically significant (P = 0.62). However, one week after the intervention, the mean score of asthma control in the intervention group was significantly less than that of the control group (P = 0.003). Similarly, at the three-month follow-up assessment, the mean score of asthma control in the intervention group was still significantly less than that of the control group (P < 0.001; Table 2).
a Values are expressed as mean ± SD.
b The results of the repeated-measures analysis of variance.
c The results of the independent-sample t test.
The variations of the mean score of asthma control in the control group were not statistically significant across the three measurement time points (P > 0.05). However, there was at least one statistically significant difference in the intervention group respecting the variations of the asthma control mean score across the three measurement time points (P < 0.05). The results of Bonferroni’s post hoc test illustrated that the differences between pretest mean score and posttest and follow-up mean scores of asthma control in the intervention group were statistically significant (P < 0.001), while there was no statistically significant difference between the posttest and the follow-up mean scores (P = 0.19; Table 3).
| Group | Pretest-Posttest | Pretest-Follow-up | Posttest-Follow-up |
|---|---|---|---|
| Intervention | -4.66 ± 3.82 | -5.22 ± 4.02 | -0.55 ± 1.74 |
| Control | -0.30 ± 1.21 | -0.08 ± 1.27 | 0.22 ± 1.41 |
| P valueb | < 0.001 | < 0.001 | 0.23 |
a Values are expressed as mean ± SD.
b The results of the independent-sample t test
Finally, the between-group differences respecting the mean difference between the pretest and the posttest and follow-up mean scores of asthma control were statistically significant (P < 0.001), while the between-group difference respecting the mean difference between the posttest and follow-up mean scores of asthma control was not statistically significant (P = 0.23).
5. Discussion
This study aimed to evaluate the effects of MI on asthma control among adolescents with asthma. The findings indicated that MI significantly improved symptoms and disease control. Although we found no similar studies for comparison, some studies were found into the effects of education on asthma control or the effects of MI on other patient outcomes. For instance, a study reported asthma education meeting to be effective in improving asthma control (7). Another study found that educations about using peak flow meter and message-based follow-up significantly improved self-management among patients with asthma (20). However, a short-term educational intervention was found to have no significant effects on asthma control and treatment adherence (3). Most educational interventions for asthma mostly focused on the enhancement of patients’ knowledge about asthma and its treatments (21). However, the present study revealed that helping patients focus more on asthma, its symptoms, and their causes could significantly improve asthma symptoms and asthma control.
Respecting the effects of MI on other patient outcomes, a study in the United States on 37 adolescents with asthma and their caregivers reported that MI significantly enhanced adolescents’ self-efficacy and motivation for receiving treatment, promoted their caregivers’ treatment adherence, and had no significant effects on adolescents’ treatment adherence (14). Another study indicated the effectiveness of MI in treatment adherence of 25 adults with asthma (13). Similarly, a study on 54 adults with asthma reported the positive effects of MI on treatment adherence even one year after the intervention (22).
The current asthma management guidelines show that the aim of treatment is to help patients effectively to control their disease (23). The MI can help the therapist to achieve this aim because it is based on sympathetic interaction, avoidance from struggling with the client, coping with his/her resistance, supporting his/her self-efficacy, and encouraging behavior modification (12). The appropriate use of MI can effectively improve asthma control. Of course, further studies with longer follow-up assessment periods are needed to provide firm evidence concerning the effectiveness of MI in improving asthma control.
Among the limitations for the present study was the lack of asthma-specific clinics in Birjand, Iran, which faced us with difficulties in accessing eligible participants for this study.
5.1. Conclusions
This study suggests the effectiveness of MI in improving disease control among adolescents with asthma. Improving adolescents’ motivation for the modification of behaviors that aggravate asthma symptoms may have considerable effects on asthma control and quality of life. Educational managers are recommended to adopt strategies to teach MI and its applications to nurses who work with adolescents who suffer from asthma.

