1. Introduction
Dilated cardiomyopathy can be primary or secondary. One of the most common causes of this dysfunction is exposure to toxic agents (such as alcohol or chemotherapy drugs) or viral and bacterial infections. However, electrolyte disturbances seem rare (1). The most common electrolyte disturbance cause after total thyroidectomy is hypocalcemia (2). Hypocalcemia can cause reduced ventricular contraction, especially dysfunction in the left ventricle (3).
In this paper, we describe the successful management of acute heart failure and dilated cardiomyopathy together with hypocalcemia and hypoparathyroidism.
2. Case Presentation
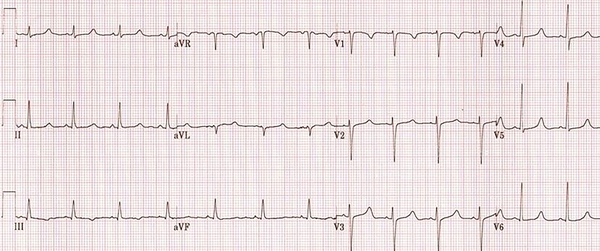
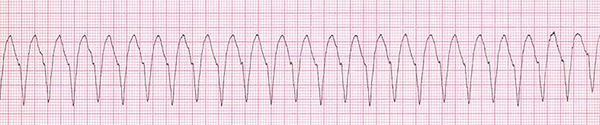
A 48-year-old woman weighted 58 kg, with a diagnosis of malignant neoplastic thyroid tumor, was admitted to the surgical intensive care unit following total thyroidectomy. The patient was complaining of worsening her general condition about one week before surgery. In the primary examination, she was in functional class 2 (NYHA Class 2) with no history of hospitalization. Also, she had not any history of family endocrine disorders. On admission, she was in a stable condition and vital signs were in normal ranges, as follows: blood pressure of 137/89 mmHg, heart rate of 89 beats per minute, and respiratory rate of 21 respirations per minute; she was also afebrile. The oxygen saturation at rest, with finger pulse oximetry in the index finger, was 96%. In the examination of the thorax, we were auscultated weak crackle in both lungs and systolic murmur with grade 2 in the apex of the heart and left the sternal border. The patient had no history of cardiac disorder when referred to a clinic for more assessment and monitoring of probable cardiac disease. The insignificant Chvostek sign was seen in the physical examination, but we could not see any evidence of the Trousseau sign. The electrocardiographic assessment on admission showed normal sinus rhythms with long QTc (510 milliseconds) (Figure 1). Immediately after the primary physical examination in the ICU, due to the matter of the surgery, we assessed the paraclinical condition of the patient before and after surgery. Electrolyte assessment before surgery showed the corrected calcium level as 8.7 mg/dL, magnesium as 2 mg/dL, and the parathyroid hormone as 10 pg/mL. Therefore, based on these data, we took a blood sample to examine the electrolyte levels after total thyroidectomy. After a while, abnormal tonic movements appeared and rapidly reduced the level of consciousness. Her heart rhythm in this period modifies to monomorphic ventricular tachycardia (Figure 2). Therefore, this life-threatening rhythm was corrected with twice 200 J defibrillated shock and the rhythm shifted to the sinus rhythm with electrical alternans immediately, based on borderline hypocalcemic condition before the surgery, and according to insignificant Chvostek sign after surgery, treatment with calcium gluconate 10% with a dose of 1 gr infusion in 5 minutes of time was initiated, and so maintenance infusion with a dose of 50 mg/kg/24 for 12 hours continued. Then, the results of laboratory assessment done before the hemodynamic disorder were reported and showed severe hypocalcemic condition (calcium level = 4.9 mg/dL). These data confirmed our accurate approach to the patient’s condition.
According to the patient in probable acute pulmonary edema condition and the severity of bilateral crackles, the furosemide as 10 mg was administrated, and then it was continued as infusion 0.5 mg/hour for 8 hours. To increase the cardiac output and considering the reduced ejection fraction, 0.25 mg digoxin was infused for 15 minutes. In her treatment regimen, we administrated captopril 25 mg daily, magnesium sulfate 1 g daily, and carbonated calcium 500 mg daily. After stabilization of the general condition and before starting the diet, an electrolyte assessment was done. The laboratory study results were as follows: corrected calcium level of 6.5 mg/dL (reference range: 8.6 - 10.2 mg/dL), magnesium level of 1.7 mg/dL (reference range: 1.6 - 3.1 mg/dL), albumin level of 3.5 g/dL (reference range: 3.5 - 5 g/dL), phosphorous level of 7 mg/dL (reference range: 2.5 - 5 mg/dL), and parathyroid hormone-I level of 9 pg/mL. Other laboratory parameters such as cell blood count, blood urea nitrogen, sodium, potassium, T3, T4, and TSH were in the normal range. Liver function tests such as alanine transaminase (SGPT) and aspartate aminotransferase (SGOT) and alkaline phosphatase was in normal range. Also, the immune assay was negative before the surgery for autoimmune antibodies and rheumatologic tests.
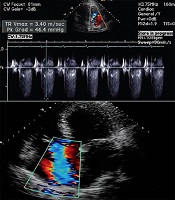
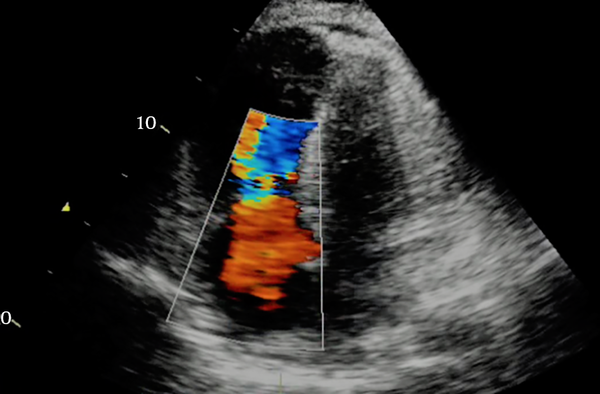
Transthoracic echocardiography immediately after the stabilization of the patient’s condition revealed hypokinetic left ventricle lateral wall with reduced ejection fraction 25%, moderate diastolic dysfunction with mild mitral valve regurgitation, and mild to moderate tricuspid valve regurgitation. The increased left side chambers of the heart were significant (Figure 3). Due to the normalization of the calcium level on the second day after the surgery (corrected calcium level 8.9 mg/dL), the patient was discharged on treatment with carbonated calcium, furosemide, and captopril. Before discharge, transthoracic echocardiography showed the improvement of left ventricular function with an ejection fraction of 40% (Figure 4).
We advised her to be evaluated for calcium level every two weeks for three months and every three months for one year after discharge and refer to a heart clinic to done serial echocardiography for close monitoring of cardiac function.
3. Discussion
Acute parathyroid insufficiency syndrome is one of the serious complications after total thyroidectomy, which may lead to hypocalcemia (4). Hypocalcemia is considered to be reversible but noncommon causes of dilated cardiomyopathy can involve any or both of the cardiac ventricles (5). This electrolyte disturbance can lead to contractility dysfunction in the heart (3), but congestive heart failure (CHF) following this disorder is very rare (6).
In the current patient, not only appeared the symptoms of CHF, but also they appeared quickly so that she showed signs of severe hypocalcemic condition only about 60 minutes after surgery. Hypocalcemia may lead to CHF and even can cause secondary ST-T changes in the electrocardiogram and manifest acute myocardial ischemia. Also, it may lead to the incidence of threatening arrhythmia such as ventricular tachycardia (7). In our patient, the increased QT interval and subsequent monomorphic ventricular tachycardia appeared rapidly.
In hypocalcemic cardiomyopathy, the enhancement of contractility and stabilization of hemodynamics are impossible with common heart failure treatment strategies. The role of calcium and vitamin D is irrefutable in the improvement of cardiac function and treatment of hypocalcemic cardiomyopathies (8). Besides, a few studies have noticed the role of the parathyroid hormone in the stabilization and maintenance of contractility of cardiac muscle (5).
The primary correction of serum calcium level is essential in the treatment of hypocalcemic cardiomyopathy, but primary treatment with this strategy is insufficient for the improvement of ventricular function and intracellular calcium correction is more effective for left ventricular ejection fraction enhancement. This issue needs the long-term use of calcium (9).
In the current patient, the treatment focused on treating acute heart failure due to electrolyte disturbances. After the primary correction of calcium level, she underwent carbonated calcium and vitamin D supplement treatment. Also, with the correct identification of the primary cause of cardiomyopathy, she did not undergo the common treatment of cardiomyopathy and this issue had a special role in the improvement of the patient’s general condition. Moreover, we advised her to monitor the serum levels of calcium to prevent the incidence of hypocalcemia; this is because, in the absence of the complete correction of intracellular calcium, cardiac muscle cell function cannot reach the stable condition, which can return the patient to the pathologic condition and the incidence of threatening dysrhythmia, especially ventricular tachycardia (10).
The physiopathology of hypocalcemia and its treatment are different in neonates and adults. In neonates, hypocalcemia is due to maternal vitamin D insufficiency and this condition is along with compensated hypoparathyroidism. In adult patients, hypocalcemic cardiomyopathy is due to hypoparathyroidism, with or without vitamin D insufficiency (11). In our patient, we prescribed vitamin D supplements along with oral calcium.
Studies showed that the addition of vitamin D supplementation to the treatment regimen of patients with heart failure significantly reduced the severity of this disorder with the reduction of ProBNP. Also, vitamin D supplementation can cause significant reductions in inflammatory agents in heart failure patients with a dramatical reduction in hs-CRP levels (12).
In our patient, there was no cause of dilated cardiomyopathy except for hypocalcemia. It implies the importance of close consideration of electrolyte disturbances, such as hypocalcemia, in thyroidectomy surgeries as the vital secondary cause of dilated cardiomyopathy. Also, the rapid and effective treatment of this disorder could improve the prognosis of the patient. The incidence of this disorder suggests the quick evaluation of patients’ electrolytes immediately after surgery to reduce life-threatening events.
The lack of ECG of the patient before the surgery was the only limitation of this report that could be effective in the accurate monitoring of the patient before, during, and after the surgery.