1. Background
The metabolic syndrome (Met.s) consists of some metabolic abnormalities such as diabetes mellitus and cardiovascular disease, which impose a remarkable effect on a health system (1). The number of individuals with metabolic syndrome is increasing, and its worldwide prevalence is estimated to be approximately 20% - 25%. The primary treatment for metabolic syndrome is the improvement of lifestyle, including modification in dietary habits, exercise, and weight loss (2).
Recently, studies focused on the potential benefit of herbal and alternative approaches for the prevention, management, and treatment of such health problems. Berberis vulgaris (barberry) Linn belongs to the family of Berberidacea that is a shrub growing in many areas of the world, including Iran (especially in Southern Khorasan Province). The fruit of Barberry has been widely used as the food additive in Iran and other countries, and it is an essential component of the Mediterranean diet. Berberine has been shown to lower elevated blood glucose as effectively as Metformin (3).
Numerous studies have outlined barberry’s properties and found that it has promising and selective anti-cancer properties; hypotensive property; beneficial effects on atherosclerosis, coronary heart disease immunity promoting effects; a preventive nature against insulin resistance, and related diseases; and hypolipidemic and antioxidant properties in animal models (4-7). Therefore, the major objective of this study was to evaluate the effect of barberry fruit on metabolic syndrome component.
2. Methods
This double-blind, randomized controlled clinical trial was conducted on patients with metabolic syndrome aged over 18 years referred to the cardiovascular outpatient clinic at Birjand University of Medical Sciences, Birjand, Iran, from May 2011 to July 2011. Metabolic syndrome was defined based on the AHA/NHLBI update of the adult treatment panel (ATPIII) (8).
Based on power 80% and α = 0.05 (2-sided), the sample size of 30 patients per group was considered. The exclusion criteria were any history of major cardiac, pulmonary, hepatic, gastroenterological, neurologic, psychiatric, or renal diseases. Also, pregnant women were excluded.
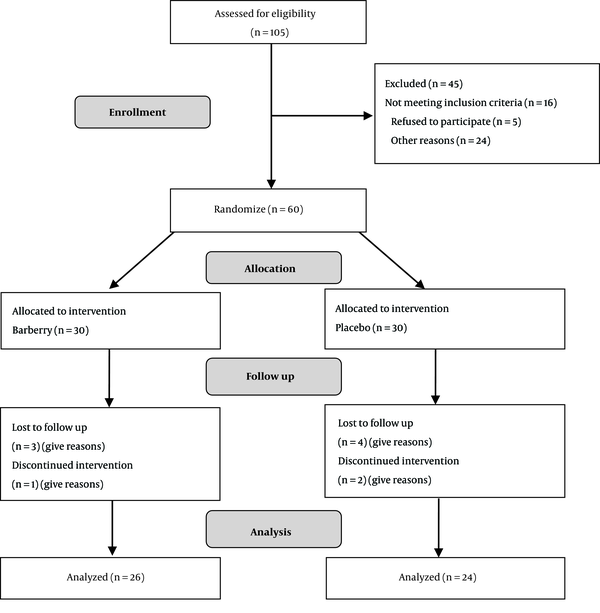
Of the 105 enrolled subjects, 50 patients completed the entire trial. All of the patients were examined by a physician to confirm the diagnosis of metabolic syndrome. The study consisted of two visits. Randomization occurred during the first visit after written informed consent was signed by the participants.
Throughout the study, the person who assessed the participants and patients were blind to assignments. All participants were randomly assigned either to be treated with barberry or to receive a placebo in a 1:1 ratio (simple random sampling). Barberry tablets were prepared by the Pharmacy School Laboratory of Mashhad University of Medical Sciences. Subjects were instructed to take one tablet daily for 21 days (Three weeks). Placebo tablets were identical in appearance to active treatments. Subjects continued consuming their usual diet; no dietary advice or guidance was provided during the study.
The study protocol was approved by the Institutional Review Boards at Birjand University of Medical Sciences and was conducted in accordance with the revised Declaration of Helsinki. This trial was registered at the Iranian Registry of Clinical Trials with the RCT code IRCT 2016112617756N11.
All participants underwent clinical evaluation. Height, weight, and blood pressure were measured according to a standard protocol by a trained health care professional. A blood sample was taken after 12 - 14 h overnight fasting and sent to the laboratory for the measurement of fasting blood sugar (FBS), serum lipids, including TG, total cholesterol (Chol), high-density lipoprotein (HDL), and low-density lipoprotein (LDL). All measurements and laboratory tests were recorded at the start of the study and after three weeks of the intervention. Subjects were requested to have no change in their lifestyle patterns during the study period.
The fruits of barberry were collected from farms of Birjand in Southern Khorasan Province, Iran. The barberry tablets were prepared as follows: at first, 4 liters of aqueous extract was made using 5 kg of dried fruits. The extract was filtered to remove particulate matter, and the remained 1500-mL of the extract was taken and concentrated in a rotary vacuum evaporator at 45°C to about 100 mL. At this time, the extract was mixed with lactose and potato starch in a common manner, granulated with water, then the moist granules dried at room temperature. The resultant mix was lubricated and subsequently compressed into tablets weighing approximately 5,500 mg (containing 75 ± 10 mg dry fruit). In addition, the placebo tablets were similar in size and color to the barberry tablets.
Data were analyzed using an independent Mann-Whitney U test and Paired t-test with SPSS (V.15) software. P < 0.05 was considered statistically significant.
3. Results
As Figure 1 shows the flow diagram of 60 patients, fifty individuals have been completed the study, a few lost to follow-up (in the barberry group n = 3 and placebo group n = 4), and dissatisfied with the assigned treatment (n = 3).
The mean age of total participants in the study was 44.0 ± 9.21 years; 47.6 ± 6.08 and 40.7 ± 10.65 years in the barberry and control group, respectively. Just over half of the participants in both study groups (60.7%) were male. In this research, all of the individuals in the control group dwelt in the city, and only one of the individuals in the barberry group dwelt in the village.
Table 1 provides the experimental data on the comparison of blood parameters before and after the treatment in the barberry and placebo group for 21 days. The results indicate that systolic blood pressure (SBP) and waist circumference were reduced significantly after using barberries (P value = 0.04). Furthermore, these results showed that the mean cholesterol level decreased after taking the placebo compared to the pre-treatment, and the difference was significant. There was no significant difference in other parameters. Interestingly, the mean level of blood hematocrit was significantly decreased after the intervention (P value = 0.004).
According to Table 2, the mean levels of cholesterol were significantly different between the placebo and barberry groups. No complication or side effect could be related to the treatment with barberry tablets.
| Berberis vulgaris | Placebo | |||||
|---|---|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | P Value Paired t-test | Pre-Treatment | Post-Treatment | P Value Paired t-test | |
| Laboratory measures | ||||||
| FBS, mg/dL | 138.8 ± 83 | 142.2 ± 103.3 | 0.67 | 124.4 ± 47.7 | 129.3 ± 75 | 0.73 |
| Chol, mg/dL | 209.4 ± 40.6 | 231 ± 45.1 | 0.07 | 232.2 ± 49.7 | 208.6 ± 41 | 0.01b |
| TG, mg/dL | 258 ± 124.6 | 271.5 ± 177.1 | 0.64 | 232.4 ± 83.2 | 197.5 ± 82.9 | 0.08 |
| HDL, mg/dL | 34.5 ± 9.6 | 39.1 ± 17.7 | 0.2 | 38.4 ± 6.8 | 38.6 ± 7.3 | 0.89 |
| LDL, mg/dL | 127.9 ± 33.6 | 137.6 ± 40.1 | 0.2 | 147.2 ± 44.9 | 127.5 ± 32.1 | 0.06 |
| Hematocrit | 45 ± 3.6 | 43.5 ± 2.7 | 0.004b | 43.5 ± 5.1 | 42.6 ± 4.7 | 0.41 |
| Blood pressure | ||||||
| SBP, mmHg | 123.1 ± 13.1 | 117.7 ± 13.2 | 0.04b | 131 ± 14 | 131.8 ± 15.5 | 0.82 |
| DBP, mmHg | 97.3 ± 102.9 | 74.8 ± 8.5 | 0.28 | 85 ± 20.6 | 84.1 ± 13.4 | 0.86 |
| Anthropometric measures | ||||||
| Waist circumference, cm | 105.9 ± 7.5 | 105 ± 7.7 | 0.04b | 101.5 ± 8.1 | 105.9 ± 15.7 | 0.15 |
| BMI, kg/m2 | 31.3 ± 3.8 | 32.7 ± 10.1 | 0.4 | 29.8 ± 5.3 | 29.6 ± 4.7 | 0.57 |
Comparison of Blood Parameters Pre and Posttreatment (after 21 days) in Barberry and Placebo Groupsa
| Berberis vulgaris (N = 26) | Placebo (N = 24) | P Value (Mann-Whitney U) | |
|---|---|---|---|
| Laboratory measures | |||
| FBS, mg/dL | -3.4 ± 39.9 | -4.9 ± 68.5 | NS |
| Chol, mg/dL | -17 ± 61.1 | 23.6 ± 45 | 0.02 |
| TG, mg/dL | -9.8 ± 146.5 | 34.9 ± 94.4 | NS |
| HDL, mg/dL | -4.3 ± 16.5 | -0.2 ± 7.3 | NS |
| LDL, mg/dL | -9.7 ± 37.6 | 19.7 ± 42.2 | NS |
| Hematocrit | 1.54 ± 2.3 | 0.9 ± 5.5 | NS |
| Blood pressure | |||
| SBP, mmHg | 5.4 ± 12.6 | -0.8 ± 17 | NS |
| DBP, mmHg | 22.5 ± 104.2 | 0.9 ± 25.1 | NS |
| Anthropometric measures | |||
| Waist circumference, cm | |||
| BMI, kg/m2 | -1.4 ± 9.4 | 0.2 ± 1.9 | NS |
Comparison of Change in Outcome Measures (after 21 days) in barberry and Placebo Groups After Interventiona
4. Discussion
Our findings indicated that tablet consumption of Berberis vulgaris (one 5,500 mg tablets per day for 3 weeks) may change some parameters in patients with metabolic syndrome. A decrease in SBP was significant after the intervention. The antihypertensive effect of barberry has been matched with those observed in some earlier animal studies. A study by Fatehi‐Hassanabad et al. (6) on hypertensive rats evaluated the effect of aqueous extract from barberry fruits and indicated that the extract significantly decreased the arterial blood pressure in rats. In vitro evaluation of aorta indicated that the antihypertensive and vasodilator effects of the extract were essentially endothelial-independent; so, they suggested that it could be used for the treatment of hypertension. This finding is in agreement with Lazavi et al. (1) findings, which showed barberry juice included some vasodilator factors like berberine. The results of this study indicated that eight weeks of consumption of this substance recovered the blood pressure and FBS in patients by releasing endothelium relaxing factor on the central nervous (CNS) system (1).
Another finding in our study was a reduction in waist circumference (abdominal obesity) but no significant decrease was observed in BMI (obesity). These results are consistent with those of other studies, indicating 24 patients with metabolic syndrome. In this Double-Blind Controlled Clinical trial, twelve of which took 3 pills (500 mg berberine) daily and the rest of the control group received the placebo for 3 months. Decreased in the SBP, waist circumference, TG, and total insulin secretion were established in their study (9). Similarly, in another clinical trial, which was conducted on 80 individuals, a significant reduction was discovered in the weight of the body, triglyceride, and cholesterol. These participants, including the case and control groups, had received two capsules (750 mg), including Berberis vulgaris extract every day for 3 months (10). Many studies confirm anti-obesity effects of berberine by examination at animals and cell culture by the prevention of adipogenesis (11). These findings have also been verified in subsequent pilot studies that administer berberine (500 mg) orally for twelve weeks in obese human subjects. A moderate weight loss (average 5 lb/subject) was the most important finding of these investigations to prove the pleasant effects of berberine juice (11).
Our findings showed that serum cholesterol decreased significantly after the intervention in the placebo group that was inconsistent with some studies such as Razzaq et al. (12) who studied the effect of barberries on lipid profile and coagulation parameters in rabbits. Zarei et al. (13) indicated that berberine reduces the density of serum cholesterol and triglycerides and can improve the function of liver enzymes. Doggrell (14) and Shidfar et al. (15) indicated that berberine significantly reduced cholesterol.
Although our results indicated that a 3-week oral consumption of barberry fruit extract had no effect on blood glucose level, some studies have reported the anti-diabetic effect of root extract on rats (7, 16). Singh and Kakkar examined the extract of Berberis aristata (root) on diabetic rats and demonstrated that it could decrease the gluconeogenesis and oxidative stress; so, it suggests a strong potential effect of root extract on the regulation of glucose homeostasis (7). Similarly, Gulfraz et al. (16) found that oral administration of 50 mg/kg ethanol root extract of Berberis lyceum in diabetic rats had a significant decrease in blood glucose levels.
Although these studies had essential differences with ours, including the use of the root plant for treating diabetes or extraction from other types of barberries, it is suggested that anti-diabetic effect of barberry fruit extract should be investigated in further clinical trials on patients with diabetes. Several limitations to this study need to be declared. The sample size is small, the current study has only examined a low dose of berberine, the duration of treatment is short, the study is bounded by the lack of review on diet and physical activity in the study groups and non-matching in laboratory features between the barberry and control groups.
4.1. Conclusions
This project was performed to evaluate the efficacy of barberry fruit in the treatment of metabolic syndrome. The results of this clinical trial show that consumption of 550 mg/per day barberry tablet had a significant effect on the reduction of SBP, waist circumference, and interestingly, decrease in hematocrit. More studies are recommended to investigate the effects of different doses of Barberry on metabolic syndrome.
4.2. Limitation
Owing to the small sample size in this study, studies with larger sample size are recommended.