1. Background
Cadmium (CD) is a toxic and hazardous metal, which is fed into the human body by agricultural, industrial, diet, and tobacco waste, causing intracellular and extracellular damage to the liver, kidneys, bones, brain, and lungs (1). The main cause of cadmium-induced kidney damage can be due to low molecular weight proteins (LMWP) disorder pathway such as b2-microglubin (b2M), retinol-bound protein (RBP), and enzymes such as N-acetyl-β-D-glucosaminidase (NAG) (2). Albumin (ALB) is a protein that attaches to the bilirubin and carries it. Increasing oxidative stress in addition to association with diabetic nephropathy and kidney-related diseases, increases bilirubin in the renal tissue (3); as a result, the researchers have reported a positive relationship between total bilirubin (TBIL) and serum ALB in patients with diabetic nephropathy (4). Unlike the antioxidant function of ALB in the blood, its elimination through the kidney demonstrates a defect in glomerular filtration and kidney failure in preventing ALB and bilirubin excretion (4). Considering the increased environmental pollution and its damaging effects on the body and heavy costs of treatment, it is important to find low-cost methods for preventing or treating kidney disease. Recent studies have shown that regular aerobic trainings can modify the metabolism of proteins in the kidneys (5). Previous studies have investigated the effects of low-intensity aerobic training on renal function, however, the results were controversial (5, 6). In regards to the controversial results about the effects of training intensity on the renal tissue, it appears that consumption of antioxidants can have beneficial effects on renal damage. For example, selenium partakes as a natural ingredient and contributor in the cofactor of several anti-oxidant and immune system enzymes (selenoproteins); consequently, the reduction of serum levels of selenium in acute and chronic kidney disease has been reported (7). Selenium seems to improve the levels of creatinine and ALB in the renal tissue of streptozotocin-induced diabetic rats, by activating and increasing the enzymatic anti-oxidant levels in the renal tissue (8). In addition, the attention of sport science researchers has recently been drawn to the use of these antioxidants, along with exercises and their interactive effects, so that a study reported the interactive effects of interval training and selenium consumption on reducing cell death markers in the liver tissue of cadmium-exposed rats (9). Furthermore, aerobic training, along with selenium consumption had antitumor induced effects on experimental mice (10). Given that the results of the studies done on the intensity and type of exercise activity on ALB, TBIL, and histidine ammonia-lyase (HAL) are contradictory, especially in the kidney tissue, researchers have not yet found the most appropriate exercise to improve the aforementioned indices in non-athlete individuals. Therefore, it seems that the findings of the present study could provide more information to sports science researchers in this field.
2. Objectives
Considering that the type of training is a factor affecting the physiological adaptations, it seems that research in this area can provide more information about the type of training in the renal tissue to prevent the damage of the kidneys caused by environmental pollution. Since environmental contamination, especially CD, can cause irreparable damage to the kidney tissue, the importance of fundamental studies (to prevent the risk of kidney disease) is high. Accordingly, the present study was conducted to review the effect of endurance training and selenium consumption on the renal tissue of cadmium-poisoned rats.
3. Methods
In this experimental trial, 40 male adult Sprague Dawley rats were purchased from the animal house of Islamic Azad University of Marvdasht Branch and transferred to the Sport Physiology Laboratory of the university. After one week of adaptation to new environment, on the eighth day, the rats were randomly assigned into eight groups of five rats, including: (1) control, (2) sham, (3) cadmium, (4) cadmium + selenium (SE), (5) cadmium + low intensity continuous training (LICT), (6) cadmium + high intensity interval training (HIIT), (7) cadmium + LICT + SE, and 8) cadmium + HIIT + SE. Rats in groups 3 - 8 received 2 mg/kg of cadmium chloride (Merk company; Germany; Cat Number: 1.02011.1000) intra-peritoneally (11); rats in groups 4, 7, and 8 received 0.23 mg/kg of selenium (Sigma-Aldrichcompany; USA; Cat Number: 10108-64-2) per day (12), and rats in groups 5 - 8 performed three sessions of selected HIIT (13) and LICT (14) per week.
3.1. Training Protocols
Initially, the maximum oxygen consumption (VO2max) was measured using Bedford et al.’s standard incremental test, evaluated by Leandro et al. (15, 16). In training groups, rats were first warmed up on the treadmill at 50% - 60% of the maximum speed for five minutes. HIIT included a combination of high intensity and low intensity interval repeats. High intensity interval repeats included two minutes at an intensity of 80% of the maximum speed in the first week, 90% of maximum speed in the second week, 100% of maximum speed in the third week, and 110% of the maximum speed from the beginning of the fourth week until the end of the training period; low intensity interval repeats (recovery interval) included 2 minutes at an intensity of 50% of the maximum speed. After performing the last high intensity interval repeat, rats performed a cool down at an intensity of 50% - 60% of the maximum speed for 5 minutes. The number of high-intensity interval repeats was determined according to the weekly training period of rats, so that in the first week, two high-intensity interval repeats; in the second week, four high-intensity interval repeats; in the third week, six high-intensity interval repeats, and from the beginning of the fourth week afterwards, it included eight high-intensity interval repeats. The total time of the training comprising high intensity and low intensity interval repeats along with warm-up and cool down, was on average 16 minutes in the first week, 24 minutes in the second week, 32 minutes in the third week, and 40 minutes from the beginning of the fourth week afterwards.
Also, rats in the LICT groups warmed up on the treadmill at an intensity of 50% - 60% of the maximum speed for five minutes. Then, they performed continuous training at an intensity of 65% of the maximum speed in the first week, 70% of the maximum speed in the second week, and 75% of the maximum speed from the third week afterwards. At the end, the rats performed 5 minutes of cool down at an intensity of 50% - 60% at a maximum speed (using a 5 channel treadmill manufactured by Daneshsalar Iranian Company).
3.2. Measurement of Research Variable
At the end of eight weeks, 48 hours after the last session of training, CA and selenium injection, the rats were anesthetized by ketamine and xylazine, and then their kidney tissue was extracted by experts to measure the variables. It is worth noting that at the end of the study, there was no drop in any of the groups.
The TBIL (MyBioSource kit; Cat Number:MBS9389057 and 0.1 µmol/L sensitivity, made in China), HAL (MyBioSourcecommercial kit; Cat Number:MBS9352178 and 1.0 ng/mL sensitivity, made in China), and ALB (Cloud-Clone Crop commercial kit; Cat Number: CEB028Ra and 0.95 µg/mL sensitivity, made in China) measured by ELISA method. To measure the research variables, based on the manufacturer’s instructions, after washing and sterilizing tissue for homogenizing excess blood was removed and weighed before homogenization. In addition, the tissues were minced to small pieces and homogenized in a certain amount of PBS (usually 10 mg tissue to 100 µL PBS). After that, homogenates were centrifuged for approximately 15 minutes at 1500 × g (or 5000 rpm).
3.3. Statistical Analysis
Shapiro-Wilk and one-way ANOVA tests were used for analysis of normality distribution of data and effect of cadmium and normal saline (selenium solvent), respectively as well as for analysis of the effect of training; in addition, selenium and their interactive effect on research variables two-way ANOVA with Bonferroni’s post hoc tests were used. The significance level was considered at 0.05.
4. Results
The levels of ALB, HAL, and TBIL in the eight study groups are presented in Table 1. The lowest ALB (26.79 ± 3.09), HAL (31.18 ± 0.84), and TBIL (21.61 ± 1.33) levels are in the control group, nevertheless, the highest ALB (74.89 ± 4.31), HAL (60.30 ± 4.02), and TBIL (31.66 ± 2.60) levels are in the cadmium group.
| Variable Group | ALB, µg/mL | HAL, ng/mL | TBIL, µmol/L |
|---|---|---|---|
| Control | 26.79 ± 3.09 | 31.18 ± 0.84 | 21.61 ± 1.33 |
| Sham | 28.78 ± 6.99 | 32.09 ± 0.62 | 21.61 ± 1.64 |
| Cadmium | 74.89 ± 4.31 | 60.30 ± 4.02 | 31.66 ± 2.60 |
| Cadmium + selenium | 57.15 ± 7.54 | 34.41 ± 1.48 | 25.27 ± 0.61 |
| Cadmium + continuous training | 55.73 ± 11.66 | 51.55 ± 2.25 | 26.77 ± 1.04 |
| Cadmium + HIIT | 54.13 ± 6.23 | 50.42 ± 3.31 | 26.79 ± 2.07 |
| Cadmium + continuous training + selenium | 43.82 ± 5.46 | 40.22 ± 1.65 | 23.98 ± 1.20 |
| Cadmium + HIIT + selenium | 51.12 ± 4.40 | 40.42 ± 1.72 | 26.09 ± 1.86 |
Levels of ALB, HAL, and TBIL in the Kidney Tissue of Rats in the Eight Study Groups
The results of the one-way ANOVA showed a significant difference in ALB (P = 0.001), HAL (P = 0.001), and TBIL (P = 0.001) between the control, sham, and cadmium groups (Table 2).
The results of Bonferroni’s post hoc test showed that there was no significant difference in the levels of ALB (P = 0.99), HAL (P = 0.99), and TBIL (P = 0.99) between the control and sham groups. However, levels of ALB (P = 0.001), HAL (P = 0.001), and TBIL (P = 0.001) were significantly higher in the cadmium group than in the control and sham groups.
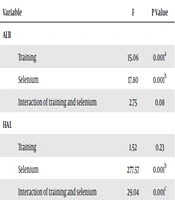
The results of the two-way ANOVA showed that training had a significant effect on the reduction of ALB (P = 0.001) and TBIL (P = 0.002) in the renal tissue of cadmium-poisoned rats; however, it had no significant effect on HAL (P = 0.23); selenium consumption had a significant effect on reduction of ALB (P = 0.001), TBIL (P = 0.001), and HAL (P = 0.001) in the renal tissue of cadmium-poisoned rats; in addition, the interaction of training and selenium consumption was significant on the reduction of TBIL (P = 0.004) and HAL (P = 0.001) in the renal tissue of cadmium-poisoned rats; however, this interaction was not significant on ALB (P = 0.08) (Table 3). The results of Bonferroni’s post hoc test showed that HIIT and LICT had a significant effect on the reduction of ALB (P = 0.001). In addition, HIIT and LICT had the same effects on the reduction of ALB (P = 0.99); moreover, HIIT (P = 0.04) and LICT (P = 0.001) had a significant effect on the reduction of TBIL, and HIIT and LICT had the same effects on reduction of TBIL (P = 0.52).
Results of the Two-Way ANOVA for Review the Effect of Selenium and Training on Research Variables
5. Discussion
Results of the present study showed that CD consumption increased levels of ALB, HAL, and TBIL in the renal tissue of rats. In the confirmation of the study, the researchers pointed out that injection of 2 mg of CD caused pathological damage to kidneys in cadmium-exposed rats’ embryos (16), in addition, exposure to CA increased inflammatory markers in patients with lung obstruction due to smoking (17), which was in line with the present study.
The results showed that HIIT and LICT had the same effects on reduction of ALB and TBIL in the renal tissue of cadmium-poisoned rats, however, HIIT and LICT had no significant effect on HAL. Since the protective role of continuous training in kidney tissue has also been demonstrated (2), the results of the present study can be justified that the antioxidant effects of long-term trainings, regardless of the intensity of training, can have favorable effects on glomerular filtration of the kidney. Although no study was found to investigate the effect of exercise on the levels of the current research variables, the researchers showed that 12 weeks of endurance training increased the serum levels of bilirubin in women (18). Furthermore, endurance training and resistance training had a significant effect on bilirubin regulation, glomerular filtration percentage, gamma-glutamyl transferase, and a significant correlation was observed in the intensity of training and increased serum levels of these markers (19). In these two studies, the tissue of measurement of the markers was not the same as the present study; however, studies have indicated that increased levels of these markers in the serum show their intestinal reabsorption and renal glomerular filtration.
The results showed that selenium consumption has a significant effect on reducing ALD, TBIL, and HAL in the renal tissue of cadmium-poisoned rats. Several studies have been conducted on the physiological effects of selenium on patients with diabetic nephropathy, cardiovascular disease, etc., however, no study was found to investigate the effects of selenium on ALB, TBIL, and HAL in the renal tissues. Nonetheless, selenium consumption was shown to improve hematological factors in patients with heart failure and ischemic heart disease (20); selenium consumption also increased serum levels of bilirubin, creatinine, malondialdehyde, and ALB, as well as decreased these markers in the renal tissue of aflatoxin-poisoned rats (21).
In addition, training and selenium consumption had interactive effects on the reduction of TBIL and HAL in the renal tissue of cadmium-poisoned rats, however, their interaction was not significant on ALB in the renal tissue of cadmium-poisoned rats. Evidence suggests that trainings with a mechanism of increasing antioxidant capacity, increasing the glomerular filtration activity, and protein reabsorption can reduce TBIL and HAL (18, 19) in the renal tissue of cadmium-poisoned rats, and the consumption of selenium by increasing the antioxidant capacity, increasing the antioxidant enzymes, and preventing the destruction of proteins prevents from kidney tubular damage (20). In this regard, the interactive effects of endurance training and selenium consumption on cadmium-induced cardiac injuries were more favorable than the effects of either one alone (14). Furthermore, aerobic training and selenium have anti-inflammatory and anti-tumor effects (10). Considering the effect size reported in the present study, it is concluded that the effect of training (effect size = 0.55) on reduction of ALB was higher than selenium (effect size = 0.42). However, the effect of selenium on reduction of HAL and TBIL (effect size = 0.92 and 0.53, respectively) was higher than training (effect size = 0.11 and 0.41, respectively). It appears that the protective effect of selenium in renal toxicity induced by cadmium is greater than training. It is worth noting that in most studies, the protective effects of exercise as well as selenium alone in the kidney tissue have been examined. However, the novelty of the present study is the simultaneous investigation as well as the interactive effects of these two factors that also lack of measurement of the estimate glomerular filtration rate (GFR), creatinine clearance, as well as pathological study were the limitation of present study. Considering changes in the percentage of glomerular filtration, creatinine, and urea-albumin levels following renal toxicity, measuring these factors could provide more comprehensive information to the researchers in this study; therefore, it is suggested that in future studies, these factors also be considered along with the variables of present research.
5.1. Conclusions
It appears that training and selenium have protective effects on renal toxicity induced by CD in rats; in addition, results of the present study showed the synergistic effects of training and selenium in protecting the kidney tissue of rats. Therefore, the combination of training and selenium consumption to prevent kidney damage due to environmental pollution is suggested.