1. Background
Physical activity (PA) is an inexpensive therapeutic strategy to prevent disease and extend healthspan (1). With increasing age, the physiological decline of muscle mass and strength must be counteracted to avoid the impairment of physical functions. Physical activity prevents functional decline among older people (2). Indeed, low-level PA may cause a vicious cycle that leads to disability and an increased incidence of adverse health outcomes. Nowadays, practicing PA as early as possible is highly recommended in the adolescent period (3). In particular, regular PA could counteract several biological characteristics of the aging process, such as oxidative stress and telomere attrition (4-6).
The telomere that is located at the end of all chromosomes is the specialized nucleoprotein structure. It acts as a protective cap and contributes to the integrity and stability of the genome (4). Telomere shortening is one of the critical hallmarks of aging and a risk factor associated with several age-related diseases such as type 2 diabetes, coronary artery disease, and Alzheimer’s disease (7).
Some mechanisms have been identified to prevent the loss of telomere length and thus compensate for telomere shortening. The ribonucleic protein-enzyme complex is the most comprehensive mechanism for telomerase extension. This complex synthesizes telomere repeats using a specific RNA template of the interval telomere sequence (6, 8). Human studies have shown that telomerase activity is vital in germline proliferating and normal somatic cells to maintain correct telomere length homeostasis, delaying senescence and tissue aging (9, 10). However, telomerase activity is influenced by various environmental factors, first of all, oxidative stress (11, 12), either in proliferating cells or in post-mitotic tissues such as skeletal muscle (13).
It is widely accepted that a well-structured protocol of PA, depending on the characteristics of exercise (i.e., type, intensity, duration, and frequency), can ameliorate the redox homeostasis of cells under physiological and pathological conditions (14, 15). On the other hand, PA reduces oxidative stress, increases telomerase activity (16), and possibly preserves telomere length.
High-intensity interval training (HIIT) is considered an adequate substitute to conventional endurance training to induce similar or even more significant changes in health and physiological performance-related markers for all populations (17, 18). A recent study demonstrated that telomerase activity did not change after HIIT protocols (short and long-term) and following four weeks of detraining. In contrast, p53 protein in HIIT with long-term interval group significantly increased in the cardiac muscle (19). It seems that increasing p53 does not affect telomerase shortening. Further, other authors have shown that short-term HIIT (eight and 12 weeks) can increase telomerase activity in peripheral blood mononuclear cells (PBMC) (20).
However, it has been shown that the HIIT-induced physiological adaptations vary depending on the number of intervals, duration, and intensity of training, and duration and activity patterns during recovery.
2. Objectives
The study aimed to examine the effects of two HIIT protocols with different work/rest intervals under a similar total time of each training session, followed by four weeks of detraining on some pathways maintaining telomere length (telomerase activity, p53 protein expression, total oxidative status (TOS), and total antioxidant capacity (TAC) levels) in rat gastrocnemius muscle. Moreover, we performed a correlation analysis among all variables to identify a possible interaction.
3. Methods
3.1. Animals and Exercise Training Protocols
Fifty-four male Wistar rats (three months old) were purchased from Birjand University of Medical Sciences, Birjand, Iran, and were randomly divided into three groups: HIIT with short-term interval (HIITSh, n = 18), long-term interval (HIITL, n = 18), and control (CT, n = 18). Rats were weighed at the beginning and end of the experimental protocols. All the experimental protocols followed the guidelines for the use and care of laboratory animals. Rats used in this study were a subset of the cohort in our recently published papers (19, 21).
We provided a regular light-dark cycle (12 : 12 h) at 22 ± 2°C in the animal house of the University of Birjand, with standard rodent chow and water ad libitum. After five days of acclimatization to treadmill running at 10 m/min for 10 min at a zero-degree incline (22), rats performed HIITSh and HIITL (at the intensity of ∼80 - 95% VO2max) five days a week for eight weeks. Before and after each training session, warm-up and cool-down were performed for five minutes at ∼ 40% VO2max (23).
The HIITSh and HIITL protocols included 16 × 1 and 4 × 4 min intervals at the intensity of ∼ 80 - 95% VO2max separated by one and four minutes active rests at 50 - 60% VO2max, respectively (19, 21, 24). The first session of HIITSh and HIITL started with eight and two work intervals, respectively, and continued as shown in Table 1. Moreover, only in the first session were rats stimulated to run on a treadmill through a mild electric shock (0.5 mA, 1 Hz) accompanied by an acoustic actuator (impact on the treadmill wall) (25). In the subsequent sessions, an auditory stimulus was used to stimulate rats.
| Weeks | High-Intensity Interval Training | |||
|---|---|---|---|---|
| HIITL | HIITSh | |||
| 1 | 8 - 12 one-minute work interval, 35 m/min | 2 - 3 four-minute work interval, 35 m/min | ||
| 8 - 12 one-minute active recovery, 16 m/min | 2 - 3 four-minute active recovery, 16 m/min | |||
| 2 | 12 - 16 one-minute work interval, 35-37 m/min | 3 - 4 four-minute work interval, 35-37 m/min | ||
| 12 - 16 one-minute active recovery, 16 m/min | 3 - 4 four-minute active recovery, 16 m/min | |||
| 3 | 16 one-minute work interval, 37 - 40 m/min | 4 four-minute work interval, 37 - 40 m/min | ||
| 16 one-minute active recovery, 16 m/min | 4 four-minute active recovery, 16 m/min | |||
| 4 - 8 | 16 one-minute work interval, 40 m/min | To end of the eighth week | 4 four-minute work interval, 40 m/min | To end of the eighth week |
| 16 one-minute active recovery, 16 m/min | 4 four-minute active recovery, 16 m/min | |||
After eight weeks of training, half of the rats in each group (n = 9) were randomly selected for sacrifice, and the other half were subjected to a detraining program for four weeks. During the detraining period, all animals had free access to food and water and were kept at rest in standard polycarbonate cages (three rats per cage). In the detraining period, two rats from the CT group and one rat from each training group died. Finally, seven rats in the CT group and eight in each training group remained and were sacrificed at the end of the detraining period.
3.2. Tissue Preparation
Forty-eight hours after the last training session and at the end of the detraining period following an overnight fast of about 12 hours, rats were sacrificed under deep anesthesia using a mixture of ketamine and xylazine (100 and 10 mg/kg body weight, respectively). Gastrocnemius muscles were removed (with appropriate surgical supplies in less than five minutes), washed with normal saline, frozen in liquid nitrogen, and stored at -80°C for further analysis.
3.3. Tissue Homogenization
Whole tissue was incised, weighed up, and lysed in phosphate-buffered saline (PBS, pH 7.4, 100 mM, 100 mg tissue/1 ml PBS) through a homogenizer. A protease inhibitor cocktail (Problock, Goldbio Inc., USA) was added to the lysis buffer to prevent protein degradation. The homogenized tissue samples were centrifuged (6,000 rpm, 10 min, 4°C). The supernatants were collected and then aliquoted for the subsequent assays.
3.4. Telomerase Activity
A polymerase chain reaction enzyme-linked immunoassay (PCR-ELISA) was used to determine telomerase activity (Telo TAGGG PCR ELISA kit, Roche, Boehringer Mannheim, Mannheim, Germany). After 20 min centrifugation at 4°C and 16,000 g, the supernatant of the tissues lysate was stored at -80°C for the sensitive protein content assay (Bradford method). The stored samples were used for the determination of telomerase activity. A telomeric repeat (TTAGGG) was briefly added to the 3′ end of a synthetic biotin-labeled primer by telomerase. Following the elongation/amplification steps, 20 µL of a denaturation solution was added, containing 5 µL of the amplification product and hybridization buffer. After two hours of incubation, 100 µL of the anti-digoxigenin peroxidase antibody reagent was added to the microplate wells, and after two hours of incubation, 100 µL of 3, 3′, 5, 5′-tetramethyl benzidine (TMB) was added as the substrate reagent. Finally, the peroxidase catalyzed reaction was arrested with an acidic stop solution. The absorbance (OD) of all wells and the reference was read at 450 nm and 620 nm, respectively. The average OD of the control group was considered 100%, and the intervened results were expressed as the ratio of the OD to the mean OD of controls.
3.5. Determination of p53 Protein, TOS, and TAC Levels
The p53 protein, TOS, and TAC levels in the supernatant of the tissue homogenates were determined using the enzyme-linked immunosorbent assay (Rat p53 ELISA kit, ZellBio GmbH, Ulm, Germany), chemical colorimetric (TOS assay kit, ZellBio GmbH, Ulm, Germany), and chemical colorimetric (TAC assay kit, ZellBio GmbH, Ulm, Germany) methods, respectively. The rat p53, TOS, and TAC assay sensitivities were 1 pg/mL, 0.5 µM, and 0.1 mM, respectively. The assays precision for p53, TOS, and TAC was 5.6%, 5.1%, and 4.6%, respectively. In addition, the total protein of the samples was assayed (Bradford total protein assay kit, ZellBio GmbH, Ulm, Germany), and the TAC, TOS, and p53 levels were expressed as µmol/mg, µmol/mg, and pg/mg protein, respectively. All kits were purchased from Padgin Teb Company (Tehran, Iran).
3.6. Statistical Analysis
The Shapiro-Wilk test was employed to check the normality assumption and Levene’s test to examine variance homogeneity. All analyses were conducted with a statistical software package (SPSS version-20 software), and data were presented as mean ± standard deviation (SD). Comparisons between groups were made using the one-way ANOVA and Tukey’s post hoc test. Given that the assumption of homogeneity of variance was violated for p53 data, the Welch method and Dunnett’s test were used for analysis. Also, the paired sample t test was used to compare intra-group weights pre and post-intervention. The Pearson correlation coefficient was used to analyze the correlation between all variables. P values ≤ 0.05 were considered statistically significant.
4. Results
4.1. Body Weight
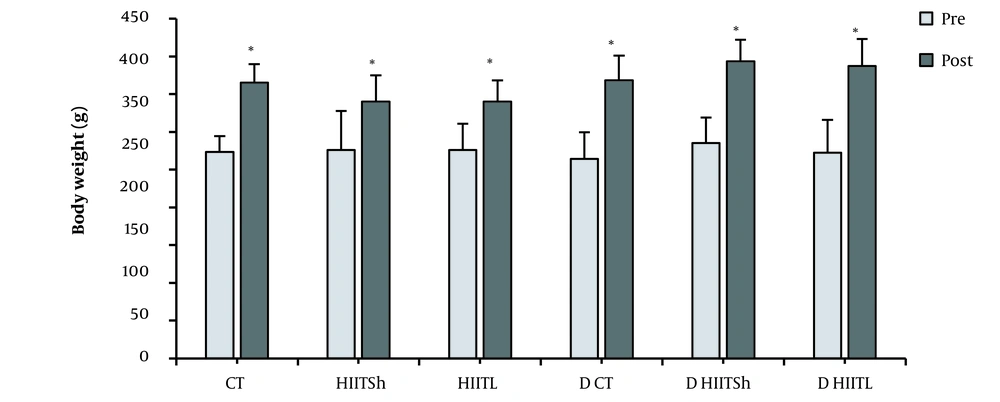
As shown in Figure 1, there was no significant difference in body weight between the groups at the beginning of the study (P = 0.91). In addition, a significant increase was observed in all groups after exercise training and detraining periods compared to pre-intervention (P ≤ 0.05). Also, there was no significant difference in body weight between the groups after the detraining period compared to the beginning of the detraining period (P = 0.09).
Body weight changes after eight weeks of high-intensity interval training and four weeks of detraining. CT, control during the training period; HIITSh, high-intensity interval training with short-term interval; HIITL, high-intensity interval training with long-term interval; D CT, control during the detraining period; D HIITSh, detraining high-intensity interval training with short-term interval; D HIITL, detraining high-intensity interval training with long-term interval. * P ≤ 0.05 compared to pre-intervention.
4.2. Telomerase Activity
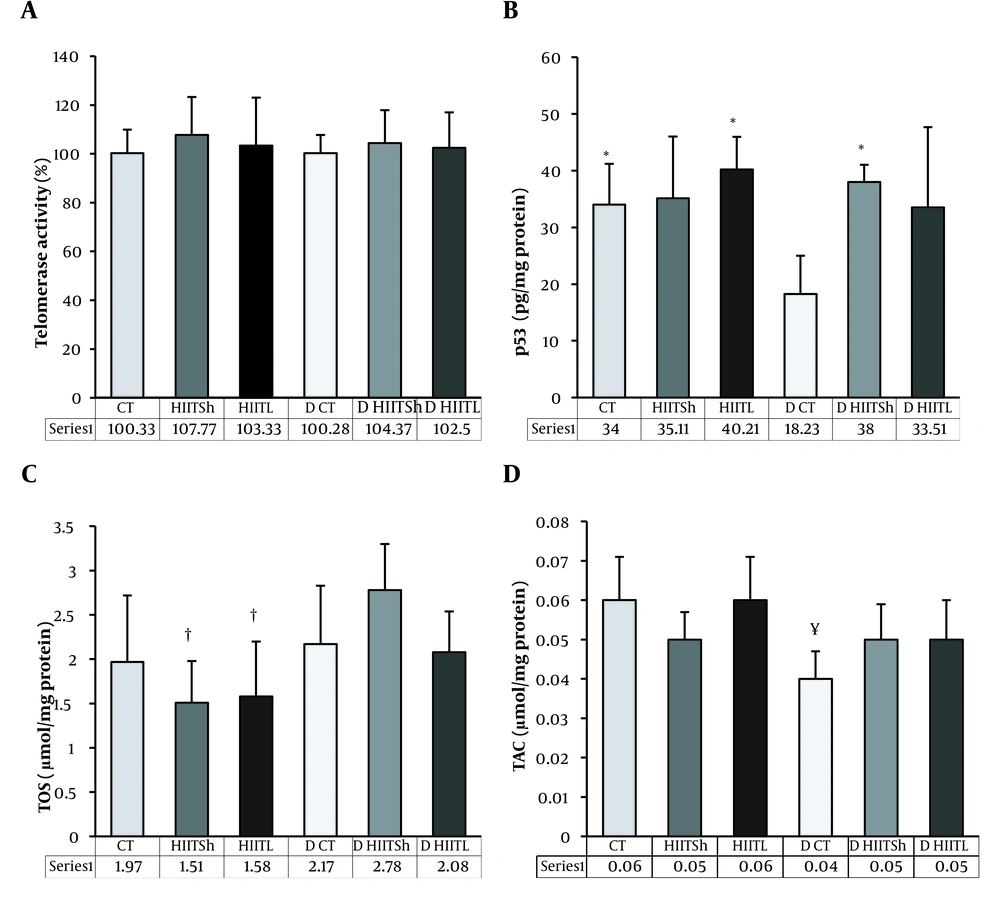
No change was found in telomerase enzyme activity after the training and detraining periods between groups (P = 0.88) (Figure 2A).
A, Telomerase activity; B, p53; C, TOS; and D, TAC levels after eight weeks of high-intensity interval training and four weeks of detraining. CT, control during the training period; HIITSh, high-intensity interval training with short-term interval; HIITL, high-intensity interval training with long-term interval; D CT, control during the detraining period; D HIITSh, detraining high-intensity interval training with short-term interval; D HIITL, detraining high-intensity interval training with long-term interval; TOS, total oxidative status; TAC, total antioxidant capacity. * P ≤ 0.05 compared to D CT, †P ≤ 0.05 compared to D HIITSh, ¥ P ≤ 0.05 compared to HIITL.
4.3. Protein Expression Level of p53
No change was found in the p53 protein levels after the training period in both HIIT groups, but a tendency to increase was observed in the HIITL group (18%) compared to the control group. However, after the detraining period, the p53 levels improved significantly in the D HIITSh group compared to the D CT group (P = 0.004) and decreased significantly in the D CT group compared to the CT group (P = 0.03) (Figure 2B).
4.4. Parameters Related to Redox Homeostasis
No change was found in the TOS level after eight weeks of training in both HIIT groups compared to the CT group, but a decreasing trend was found in the HIITSh (23%) and HIITL (20%) groups compared to the CT group. In addition, the TOS level was significantly higher in the D HIITSh group than in the HIITSh and HIITL groups (P = 0.001 and P = 0.002, respectively) (Figure 2C).
The results also revealed no significant change in the TAC level during the training period in both HIIT groups, but it was significantly lower in the D CT group than in the HIITL group (P = 0.023) (Figure 2D).
4.5. Associations Between Variables
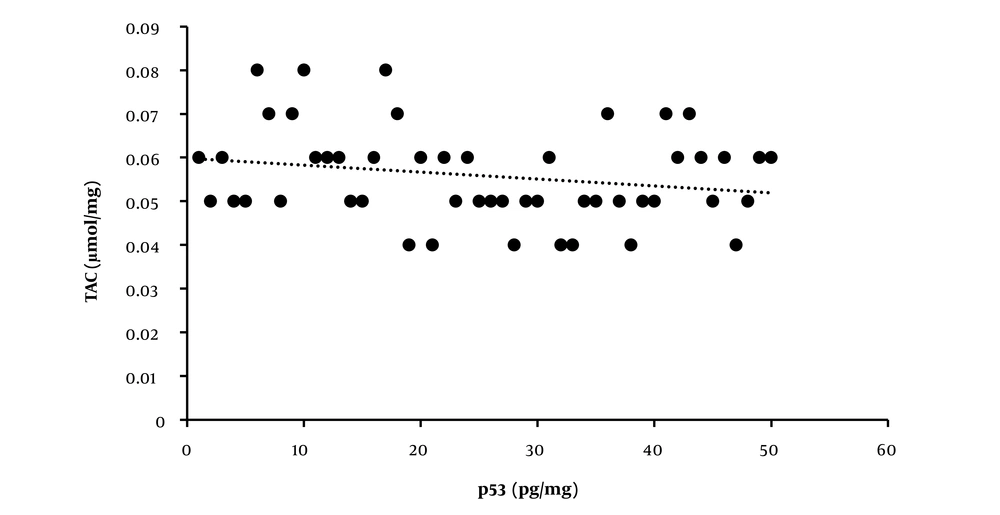
The correlations between variables are reported in Table 2. The positive associations between the p53 and TAC levels are shown in Figure 3. However, no association was found between telomerase enzyme activity and p53, TAC, and TOS levels. There was also no association between TAC and TOS.
| Telomerase Activity | p53 | TAC | TOS | |
|---|---|---|---|---|
| Telomerase activity | - | 0.18 A | 0.01 A | -0.18 A |
| - | 0.25 B | 0.94 B | 0.19 B | |
| p53 | 0.18 A | - | 0.66 A | 0.18 A |
| 0.25 B | - | 0.0001 B, C | 0.24 B | |
| TAC | 0.01 A | 0.66 A | - | 0.17 A |
| 0.94 B | 0.0001 B, C | - | 0.22 B | |
| TOS | -0.18 A | 0.18 A | 0.17 A | - |
| 0.19 B | 0.24 B | 0.22 B | - |
Abbreviations: TOS, total oxidative status; TAC, total antioxidant capacity.
a For each comparison between the indicated variables, A in superscript indicates the Pearson correlation value, while B in superscript indicates the P-value and C in superscript highlights significant correlations (P ≤ 0.05).
5. Discussion
Many studies have introduced telomerase enzyme activity as a potent indicator of telomere length to examine cellular viability or genomic stability and disease processes (26). Similar to Radak et al.’s results (27), we found that both HIIT protocols did not change telomerase activity. As suggested by others (27), we hypothesized that eight weeks of these exercise protocols are not enough to modify the telomerase activity. Indeed, Ludlow et al. found that only when the exercise training program was sustained for a more extended period (≈ one year), telomerase activity was significantly increased in rat skeletal muscle (28).
Moreover, it has been suggested that a reduction or inactivation of telomerase activity plays a direct causal role in mammalian aging (29, 30). Therefore, given the possibility that telomerase activity is reduced in elderly subjects/animals compared to young subjects/animals, the importance and role of these exercise protocols in the development of telomerase activity in older rats can not be denied. However, estimation of enzymatic activity modification at optimal levels could be more challenging in young subjects. In support of this assumption, Osthus et al. demonstrated that older endurance athletes (66 - 77 years) had improved telomeres homeostasis compared to older people with lower physical activity levels. Differences were observed between young endurance athletes (22 - 27 years) and young non-athletes (26).
Often referred to as the “Guardian of Genome,” p53 is a master regulator of genome integrity to activate the transcription of many essential genes for cell cycle control and apoptosis followed by DNA damage (28-31). Moreover, p53 binds to several loci in the cellular genome potentially, which may not be associated with transcription control. This results from a reported genome-wide scan study about p53 (32). In particular, Tutton et al. showed that p53 could bind the sites in the subtelomeric region close to the terminal telomere repeat tracts. They proposed that p53 binding to these regions confers local chromatin changes associated with increased genome stability (33). Considering that p53 is also implicated as a critical modulator of skeletal muscle in exercise-induced mitochondrial biogenesis and substrate metabolism (34), many studies have aimed to identify exercise prescription guidelines to target p53 signaling strategically.
Similar to the results of Safdar et al. (35), no change in the p53 protein levels was observed following both models of HIIT in our study. However, considering the mean values of changes, we found that HIITL increased the protein level of p53 by 18%. Ludlow et al. observed increased expression of the p53 gene in skeletal muscle after one year of exercise (voluntary wheel running) (28). On the other hand, they found that the expression of the p53 gene declined over time (with age).
From our point of view, we think that the training period and age of animals used in the study could be important factors in the results obtained from the present study. However, it must be considered that compared to control animals (CT group), the training period keeps the expression of p53 protein more stable, even during the post-training period. Therefore, although the training does not significantly increase the p53 levels in muscle cells, HIIT is likely to reduce cellular impairment and delay muscle aging by maintaining its level in the cell. This effect probably will remain with detraining.
Oxidative stress can be caused by excessive reactive oxygen species (ROS) production, leading to DNA damage and senescence or apoptosis (36). Several authors have shown that oxidative stress is closely correlated with altered telomeres homeostasis (37). Generally, regular exercise can protect telomeres from shortening by reducing oxidative stress levels (38). In the present study, we observed that TOS levels in the HIIT groups decreased compared to the control group (according to the change of mean values, a decrease of 19% in the HIITL group and a decrease of 23% in the group HIITSh) with no change in TAC after the training. Many studies have reported the effect of exercise training on the oxidant-antioxidant system. De Araujo et al. showed that oxidative stress temporarily increased after six weeks of HIIT; then, it decreased after 12 weeks of continuous training; but they did not see any significant changes in the antioxidant system in the gastrocnemius muscle of Wistar rats, which was attributed to the insignificant disturbance of ROS (39). Based on the results of de Araujo et al. (39), if HIIT continues for a more extended period, it will probably exhibit significant effects on the TOS level. On the other hand, it is accepted that a higher level of ROS leads to more adaptation in the antioxidant system (40). In this regard, Hyatt et al. displayed that 10 days of treadmill training enhanced the level of the antioxidant index in the heart and plantaris muscles, while no significant changes were observed in the soleus muscle. The researchers stated that different antioxidant adaptations in the plantaris, heart, and soleus muscles are related to the primary content of intrinsic antioxidants before training or oxidative stress levels owing to exercise. It has been found that the fast-glycolytic muscle fiber phenotype in the plantar may tolerate a higher level of oxidative stress and be replaced by rising antioxidant proteins to a greater degree than the more oxidative-fiber types of soleus and cardiac muscle (41).
Given that the gastrocnemius is an interstitial muscle (a mixture of fast and slow-twitch fibers), it is likely that in the present study, the training-induced ROS in the gastrocnemius muscle did not increase to such an extent to cause significant changes in antioxidant capacity. Indeed, after eight weeks of training, a reduction in oxidative stress was observed, probably indicating that training altered the antioxidant content, but it was not potent enough to increase TAC. Watson et al. showed that the levels of uric acid, β-carotene, and glutathione (GSH) grew considerably following regular exercise, while TAC declined meaningfully. The lack of connotation between internal antioxidants and TAC was attributed to obstacles in the TAC analysis (42). However, a review of studies conducted by others determined contrary findings in this regard. We can refer to the investigated tissues, the time course, the type (43), and the intensity of exercise training protocols to explain inconsistencies in existing reports.
Concerning the effect of detraining on oxidative stress and antioxidant markers, Fatouros et al. demonstrated that endurance training possibly diminishes basal and exercise-induced lipid peroxidation and enhances protection against oxidative stress by rising TAC. It is noteworthy that detraining may converse these training-induced adaptations (44). Radak et al. also proposed that the advantageous effects of training can change due to detraining (45). Sheikholeslami-Vatani et al. showed that sprint exercise training could induce adaptations in lipid peroxidation and the antioxidant system, which would be reversed due to detraining (40). There is no agreement on the place of ROS production in skeletal muscle during detraining periods. In this regard, Whidden et al. proposed the xanthine as a possible source of oxidants in rat skeletal muscle through lengthy periods of inactivity (46). On the other hand, Kavazis et al. propounded that mitochondria are a significant source of ROS production in skeletal muscle during inactivity (47). Sheikholeslami-Vatani et al. identified that injuries imposed due to the training could increase ROS during detraining (40). In the present study, it was observed that during the detraining period, there was a tendency for the TOS level to increase so that the increased TOS level was significantly higher in the detraining HIITSh group than in both HIIT groups. This can be confirmed by other studies due to the deletion of adaptations to the antioxidant capacity during training cessation. Overall, exercise training may act as a stimulus for reducing oxidative stress, primarily when performed continuously. Nevertheless, the effects of HIITL are more stable. However, more studies are required to confirm these results.
In this study, we identified a significant positive association between the levels of p53 and TAC. However, our results did not show any significant correlation between other variables. Therefore, HIIT could help prevent muscle aging by increasing TAC.
5.1. Limitations
This study did not examine the oxidants and antioxidants content and other possible mechanisms affecting telomere length maintenance. This is while the oxidants and antioxidants content could more accurately indicate the possible effects and differences of these two protocols and increase the general knowledge of HIIT. In this regard, detailed studies must be performed to assess the effect of long periods of the two investigated protocols and subsequent detraining on other pathways affecting the telomere length maintenance and oxidant and antioxidant content.
5.2. Conclusions
Telomeres and mitochondria are known as the main factors controlling cellular aging. According to this study, short-period exercise training does not change telomerase activity in rat skeletal muscle, and the time course is an essential factor in increasing the activity of skeletal muscle telomerase in response to exercise training. However, short-period exercise training may help maintain telomere length through other pathways such as attenuation of oxidative stress. The time of the work intervals and the recovery periods between intervals of HIIT induce different and persistent effects. High-intensity interval training with long-term intervals would be more effective because of adaptations in the pathways maintaining telomeres length (attenuation of oxidative stress) and improving the mitochondrial function and content (possibly by increasing p53 levels) with persistent, lasting effects on controlling muscle aging.