1. Background
Chronic kidney disease (CKD) is a public health problem, with a global prevalence of nearly 850 million, which is 20 times more common than HIV and twice the estimated global prevalence of diabetes (1). It is projected to become the fifth leading cause of death in the world by 2040 (2), and is considered the epidemic of the 21st century due to rising morbidity and mortality rates (3). In 2017, 12 million people lost their lives due to CKD, and 6975 million cases of all-stage CKD were recorded, for a global prevalence of 91% (4).
CKD affects between 10 to 16% of the general adult population in Asia, Australia, Europe, and North America (4). In Africa, the overall prevalence in the general population was 15.8% for CKD stages 1 to 5, and 4.6% for CKD stages 3 to 5 (5). Due to the increased prevalence in Africans of known risk factors for CKD such as diabetes, hypertension, genetic polymorphisms, like apolipoprotein L1, and sickle cell trait, African descendants are at high risk of developing CKD and progressing to end-stage renal disease (ESRD) (5-7).
The prevalence of CKD in people with diabetes was at 13% in sub-Saharan Africa and ranged from 11 to 20% in North Africa (8). While the prevalence of CKD was 4% in Egypt in 2016, it was 7.4% in Morocco (5).
Morocco is a low- or middle-income country (LMIC) where the demographic transition resulting from population aging, urbanization, and the global diabetes epidemic is exposing an increasing number of people to CKD (9). According to the results of the MAREMAR (Maladie Rénale Chronique au Maroc) study: The prevalence of CKD, hypertension, obesity, and diabetes is 6.6%, 28.2%, 24.2%, and 32.8%, respectively (10).
Diabetes and hypertension, and vascular diseases are the two major underlying End-Stage Renal Disease (ESRD) in Morocco (9), where hemodialysis (HD) becomes a necessity in renal replacement therapies (RRT) (11). According to the Moroccan Society of Nephrology, the number of ESRD patients treated by hemodialysis has increased from about 7000 patients in 2008 to 30000 in December 2018 (12).
The dialysis population is growing rapidly worldwide, and the majority (approximately 89%) of these patients are on hemodialysis (HD) (13), which is the commonest form of RRT in the world (14).
In Morocco, it is the most prevalent modality of RRT (99%) (9). Certainly, there are treatment methods for HD that ensure patients’ comfort and improve their life expectancy. However, HD is still a heavy alternative, which considerably affects both the physical and mental quality of life (QoL) of end-stage renal failure patients (15). Thus, the QoL of ESRD patients must be explored and measured in order to better identify and monitor the conditions of patients.
QoL has emerged as an important concept and an indispensable target in health and medical research and practice. Understanding QoL is an important step in promoting patient care and rehabilitation to alleviate symptoms and adjust ineffective therapies (16). QoL in health is a specific approach that covers individual satisfaction and well-being in front of the disease, treatment, and health condition (17).
QoL is also important for identifying discomforts and concerns that may affect patients, which can be communicated to future patients and allow them to anticipate the consequences of their disease and the discomforts of their treatment (16), particularly QoL is still of high importance for dialysis, given the profound effect of hemodialysis on patients' daily lives.
Studies have consistently shown that hemodialysis patients suffer from a lower level of health-related quality of life (HRQoL) and life expectancy compared to the general population (5, 10, 15, 18), due to vulnerability and deficit of physical, mental, and social well-being (19). Poor QoL is strongly associated with an increased risk of morbidity and mortality in ESRD (20). However, in Asia and Africa, the Qol of HD patients is poorly studied, particularly in LMIC (19).
In Morocco, few studies have addressed the QoL of hemodialysis patients. Notably, a recent study conducted by Chrifi Alaoui et al. (21), which intended to compare the QoL of patients that undergone HD and peritoneal dialysis, and the study of Bouidida (22), which focused on the translation and validation of KDQoL-SF v 1.3 in dialectal Arabic. In Morocco, particularly in the Souss Massa region (where hemodialysis patients face several constraints), factors associated with QoL in ESRD patients have not been studied.
According to a recent study conducted in the region of Souss Massa (23), 54% of hemodialysis patients are inactive, 10% are disabled, more than 62% of patients use a common means of transportation, and the majority suffer from transportation costs and direct expenses related to external assessment and imaging. This can sometimes cause renunciation of dialysis care. In the Souss Massa region, despite free dialysis sessions in the public sector, hemodialysis patients still face difficulties in terms of geographical access to dialysis care, and enormous financial and social difficulties (lack of stable income), which inevitably affect their QoL. Thus, investigating sociodemographic, clinical, and biological factors associated with QoL of hemodialysis patients in Morocco, particularly in the Souss Massa region, is of high importance and a necessary task. Knowledge and learning about these factors not only are essential to improve patients’ care but also help the most vulnerable and pave the way for developing appropriate interventions (11, 24). Public health interventions should take into account all aspects affecting the QoL of hemodialysis patients.
Additionally, the level of professional qualification and experience of the medical and nursing staff can influence the QoL of hemodialysis patients (25); hence, nephrologists and nurses should assimilate and understand all factors that can affect the QoL of this vulnerable population, knowing that one of the major goals of CKD management is to implement interventions that improve HRQoL (11).
In this context, we conducted this multicenter study on a relatively large sample. According to the best knowledge of the authors, this is the first study in the Souss Massa region that investigated HRQoL and KDQoL in the hemodialysis population, without forgetting associated social, demographic, clinical, and biological factors. Also, the present study was based on KDQoL-SF 36 Tm scale, which is a widely accepted assessment tool to assess QoL in hemodialysis patients.
2. Objectives
In our study, we will investigate HRQoL and KDQoL and explore their associated factors among hemodialysis patients in the Souss Massa region in Morocco using the short form of kidney disease (KDQoL-SF™ version 1.3).
3. Methods
3.1. Ethics Approval and Consent
The ethics committee of biomedical research of Mohamed the Vth University of Medicine and Pharmacy of Rabat has approved this study (N/R: Case number 11/20). Confidentiality of information was respected, and all participants provided and signed written informed consent.
3.2. Study Design and Settings
Following a cross-sectional design, this study was conducted on 441 hemodialysis patients from February-September 2020. The participants were recruited from all public-sector hemodialysis centers (9 centers) in the Souss Massa region, Morocco. The study was carried out in all dialyses centers (9 centers) under the public sector in the Souss region of Morocco: Dialysis center of Agadir regional hospital, dialysis center of Inzegane provincial hospital, dialysis center of Ait Melloul, dialysis center of Eljihadia, dialysis center of Taroudant provincial hospital, dialysis center of Oulad Taima, dialysis center of Biougra provincial hospital, dialysis center of Tiznit provincial hospital and dialysis center of Tata provincial hospital.
3.3. Study Participants
All hemodialysis patients (n = 441), recruited from all public-sector hemodialysis centers (9 centers) in the Souss Massa region, met the following inclusion criteria: Age ≥ 18 years old, duration of hemodialysis ≥ 3 months, lack of recent change in usual lifestyle habits, and prior agreement. Patients unable to respond and/or in a coma, patients with psychiatric disorders, and non-consenting were excluded from the study.
3.4. Instruments and Measurements
After a review of several articles on this topic and in consultation with experts in this field, a questionnaire was designed to collect sociodemographic data, which included age and sex, health insurance status, education level, marital status, occupation, living conditions, residence, and medical bills. The following anamnestic data were collected using the medical records: Comorbidities, toxic habits, and compliance with hygienic-dietary rules; clinical and dialytic data included: Body mass index, number of dialysis sessions per week, duration of hemodialysis, inter dialytic weight gain, and vascular approach; and biological data included: Hemoglobin (Anemia is defined as Hgb < 11 g/dL) (26), phosphoremia, albumin, blood calcium, and thyroid workup.
Participants’ QoL was studied using the Kidney Disease Quality of Life Short Form: KDQoL-SF 36 Tm (version 1.3) (27), with the validated Moroccan version (22). In fact, the KDQoL-long form is the first version of the KDQoL, which has 134 items covering 11 kidney disease-specific scales, as it is a long questionnaire, it often results in insufficient responsiveness. Thus, the KDQoL-SF v.1.3 is the most suitable measurement tool for large-scale assessments in dialysis centers, and it is the most widely used standard tool for determining QoL in CKD patients worldwide (25, 27). This can be attributed to its ease of administration and relatively low burden on patients and staff.
This commonly used instrument is specifically developed for people with kidney disease and on dialytic treatment (27). It consists of two parts. The first part, which is the generic core (HRQoL), intends to assess the general state of health of hemodialysis patients and has two dimensions: Mental component summary (MCS) and physical component summary (PCS). It contains 36 items in different areas: Physical function (10 items), general health (5 items), pain (2 items), role function (4 items), social functioning (2 items), well-being and role (3 items), physical role (5 items), and energy / tiredness (4 items) (27, 28). Two summary scores are generated: The physical component summary (PCS) and the mental component summary (MCS). The HRQoL score was obtained using the mean value of the two obtained scores.
The second part, which is a specific core (KDQoL) about renal failure, is a kidney disease component summary (KDCS) (28), targeting the particular concerns of people with kidney disease and on dialysis to assess CKD-specific QoL. It contains 43 items across different domains: Symptoms / dysfunctions related to the renal failure scale (12 items), effects / charges in connection with the renal failure scale (8 items), sleep (4 items), the burden of kidney disease (4 items), quality of social interactions (3 items), work status (2 items), sexual (2 items) and cognitive function (3 items), dialysis staff encouragement (2 items), social support (2 items), and patient satisfaction (1 item) (28). The items were recoded to be between zero (the lowest) and 100 (the highest) to calculate the subscale scores, with higher scores representing better QoL. Then, the average of the items belonging to the same scale was calculated to obtain the KDCS score, which is the KDQoL score. The scores were calculated following the user manual (27).
Following KDQoL guidelines (29), using the mean and standard deviation (SD) for the HRQoL score and the KDQoL score, three categories of QoL level were defined for HRQol and KDQol: Poor, moderate, and good. A level below the mean-1SD (standard deviation) was considered poor QoL; a level equal to the mean ± SD was considered moderate QoL, and a level above the mean + 1 SD was considered good QoL.
3.5. Steps of Data Collection
Once permission to access the dialysis centers was granted by the regional health director, the managers of these units were contacted and informed of the study protocols with detailed explanations, and appointments for data collection were made. To avoid participant fatigue, they were asked to participate in the study and provide information before their hemodialysis session began. All eligible patients who were willing to participate were invited to complete the survey after providing them with all essential information regarding the study. During the face-to-face interviews, the questionnaire was administered by the investigator of this research; on average, each interview lasted approximately 25 minutes.
3.6. Data Management and Statistical Analysis
The factors associated with HRQoL and KDQoL were determined by univariate and multivariate logistic regression. The independent variables with a P value less than 0.25 in the univariate analysis were all included in the multivariate logistic regression analysis. For any P < 0.05, the difference is considered statistically significant. Data analysis was administered by the SPSS software package for Windows (ver. 13.0; SPSS Inc., Chicago, IL, USA). The pseudo-R-squared test and the Hosmer-Lemeshow chi-square test were used to check the model fit before interpreting the final model.
4. Results
4.1. Characteristics of the Participants
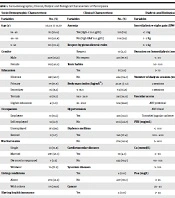
Four hundred and forty-one chronic hemodialysis patients were studied. The mean age of participants was 56.05 ± 15.67, while the median was 58 years (IRQ 45 - 68). One hundred and thirty-five patients (30.6%) are elderly (> 65 years). One hundred and ninety-two patients (43.5%) were female. Four hundred and thirty-eight patients (99.3%) were from urban areas, and only 32 (7.3%) were covered for medical costs. Sociodemographic characteristics of patients are summarized in Table 1.
| Socio-demographic Characteristic | Clinical Characteristic | Dialytic and Biological Characteristic | |||
|---|---|---|---|---|---|
| Variables | No. (%) | Variables | No. (%) | Variables | No. (%) |
| Age (y) | 56.05 ± 15.67 | Anemia | Interdialytic weight gain (IDWG) | 2.38 ± 1.05 | |
| 18 - 45 | 113 (25.6) | Yes (Hgb < 11.0 g/dL) | 335 (76) | < 1 kg | 205 (46.5) |
| 46 - 65 | 193 (43.8) | No (Hgb ≥ 11.0 g/dL) | 106 (24) | 1 - 2 Kg | 155 (35.1) |
| > 65 | 135 (30.6) | Respect hygieno-dietetic rules | > 2 kg | 81 (18.4) | |
| Gender | Respect | 10 (2.3) | Duration on hemodialysis (mon) | 64.84 ± 49.67 | |
| Male | 249 (56.5) | No respect | 431 (97.9) | < 50 | 208 (47.2) |
| Female | 192 (43.5) | Toxic habits | 50 - 100 | 152 (34.5) | |
| Education | Yes | 17 (3.9) | > 10 | 81 (18.4) | |
| Illiterate | 281 (63.7) | No | 424 (96.1) | Number of dialysis sessions /week | |
| Primary | 79 (17.9) | Body mass index (kg/m²) | 23.18 ± 3.6 | 2 sessions | 399 (90.5) |
| Secondary | 36 (8.2) | < 18.5 | 74 (16.8) | 3 sessions | 42 (9.5) |
| Tertiary | 41 (9.3) | 18.5 - 24.9 | 241 (54.6) | Vascular access | |
| Higher education | 4 (0.9) | 25 - 29.9 | 126 (28.8) | AVF proximal | 131 (29.7) |
| Occupation | Hypertension | AVF distal | 293 (66.4) | ||
| Employee | 51 (11.6) | Yes | 235 (53.3) | Tunneled jugular catheter | 17 (3.9) |
| Self employed | 12 (2.7) | No | 206 (46.7) | PTH (mg/mL) | 476.17 ± 216.97 |
| Unemployed | 375 (85) | Diabetes mellitus | < 300 | 136 (30.8) | |
| Retired | 3 (0.7) | Yes | 165 (37.4) | 300 - 600 | 214 (48.5) |
| Marital status | No | 276 (62.6) | > 600 | 91 (20.6) | |
| Single | 61 (13.8) | Cardiovascular diseases | Ca (mmol/L) | 51.05 ± 31.46 | |
| Married | 297 (67.3) | Yes | 19 (4.3) | < 90 | 393 (89.1) |
| Divorced or separated | 7 (1.6) | No | 422 (95.7) | 90 - 105 | 40 (9.1) |
| Widower | 76 (17.2) | Systemic diseases | > 105 | 8 (1.8) | |
| Living conditions | Yes | 4 (0.9) | P04 (mg/L) | 46.83 ± 16.48 | |
| Alone | 270 (61.2) | No | 437 (99.1) | < 25 | 17 (3.9) |
| With others | 171 (38.8) | Cancer | 25 - 45 | 247 (56) | |
| Having health insurance | Yes | 4 (0.9) | > 45 | 177 (40.1) | |
| Yes | 431 (97.9) | No | 437 (99.1) | Albumin (g/ L) | 42.75 ± 14.68 |
| No | 10 (2.3) | Liver diseases | < 38 | 136 (30.8) | |
| Place of residence | Yes | 3 (0.7) | 38 - 50 | 214 (48.5) | |
| City | 438 (99.3) | No | 438 (99.3) | > 50 | 91 (20.6) |
| Village | 3 (0.7) | ||||
| Support for medical costs | |||||
| With support | 32 (7.3) | ||||
| Without support | 409 (92.7) | ||||
Abbreviations: OR, odds ratio; CI, confidence interval.
The mean body mass index (BMI) was 23.88 ± 3.40 kg/m2. The mean duration of hemodialysis was 64.80 ± 49.71 months, and 335 participants (76%) were anemic. Forty-two patients (9.5%) do three sessions per week, while the majority (90.5%; n = 399) do two dialysis sessions per week. All collected clinical, dialytic, and biological parameters are summarized in Table 1.
4.2. Quality of Life
For HRQoL, the global mean score (SMG) was 40.27 ± 10.27 and for KDQoL was 40.74 with a standard deviation (SD) of 9.44 (Table 2). In summary, the participants' QoL in the two cores of the KDQOL-SF 36 Tm were as follows: 29.5% (n = 132) of participants had a poor HRQOL, and 21.3% (n = 94) had poor KDQoL (Table 3).
| Module | Mean ± SD | MIN | MAX |
|---|---|---|---|
| Generic score HRQOL | 40.27 ± 10.27 | 12.30 | 75 |
| Specific score KDQOL | 40.74 ± 9.44 | 16.51 | 80.42 |
Abbreviation: SD, standard deviation.
| Total | Level of QOL | |||
|---|---|---|---|---|
| Poor Level (< Mean-1SD) | Moderate Level (Mean+/−1SD) | Good Level (> Mean+1SD) | ||
| Generic score HRQOL | 441 | 130 (29.5) | 214 (48.5) | 97 (22) |
| Specific score KDQOL | 441 | 94 (21.3) | 271 (61.5) | 76 (19.2) |
Abbreviation: SD, standard deviation.
4.3. Factors Associated with Poor HRQoL and Poor KDQoL on Univariate and Multivariate Analysis
4.3.1. Univariate Analysis
Sociodemographic factors associated with poor HRQoL and Poor KDQoL on univariate analysis are presented in Table 4.
| Factors | Poor HRQOL | Poor KDQOL | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P-Value | OR | 95%CI | P-Value | |
| Age (y) | ||||||
| 18 - 45 | 1.48 | 0.83 - 2.64 | 0.182 | 1.02 | 0.52 - 2.02 | 0.938 |
| 46 - 65 | 0.82 | 0.51 - 1.33 | 0.349 | 0.50 | 0.28 - 0.87 | 0.015 |
| > 65 | 1 | / | / | 1 | / | / |
| Gender | ||||||
| Male | 1.11 | 0.73 - 1.61 | 0.613 | 1.06 | 0.67 - 1.67 | 0.801 |
| Female | 1 | / | / | 1 | / | / |
| Education | ||||||
| Illiterate | 0.12 | 0.07 - 6.74 | 0.751 | 0.12 | 0.07 - 6.74 | 0.901 |
| Primary | 0.09 | 0.08 - 8.41 | 0.877 | 0.09 | 0.08 - 8.41 | 0.956 |
| Secondary | 0.29 | 0.13 - 14.91 | 0.780 | 0.29 | 0.13 - 14.91 | 0.701 |
| Tertiary | 0.06 | 0.14 - 32.52 | 0.576 | 0.06 | 0.14 - 32.52 | 0.998 |
| Higher education | 1 | / | / | 1 | / | / |
| Occupation | ||||||
| Employee | 5.87 | 0.43 - 79.77 | 0.183 | 25 | 1.11 - 561.28 | 0.043 |
| Self employed | 6.00 | 0.25 - 140.04 | 0.265 | 6 | 0.25 - 140.04 | 0.265 |
| Unemployed | 1.00 | 0.09 - 11.22 | 0.995 | 1.55 | 0.13 - 17.34 | 0.720 |
| Retired | 1 | / | / | 1 | / | / |
| Marital status | ||||||
| Single | 1.77 | 0.83 - 3.79 | 0.137 | 0.90 | 0.41 - 1.95 | 0.801 |
| Married | 1.28 | 0.75 - 2.18 | 0.351 | 1.31 | 0.72 - 2.37 | 0.363 |
| Divorced or separated | 0.70 | 0.14 - 3.38 | 0.663 | 1.93 | 0.21 - 17.08 | 0.554 |
| Widower | 1 | / | / | 1 | / | / |
| Living conditions | ||||||
| Alone | 1.37 | 0.90 - 2.08 | 0.135 | 1.37 | 0.90 - 2.08 | 0.357 |
| With others | 1 | / | / | 1 | / | / |
| Having health insurance | ||||||
| Yes | 0.97 | 0.24 - 3.82 | 0.971 | 2.47 | 0.31 - 19.79 | 0.393 |
| No | 1 | / | / | / | / | / |
| Place of residence | ||||||
| City | 0.83 | 0.07 - 9.28 | 0 .883 | 0.53 | 0.04 - 6.01 | 0.616 |
| Village | 1 | / | / | / | / | / |
| Support for medical costs | ||||||
| With support | 0.78 | 0.36 - 1.67 | 0.529 | 0.41 | 0.19 - 0.89 | 0.024 |
| Without support | 1 | / | / | 1 | / | / |
Abbreviations: OR, odds ratio; CI, confidence interval.
Clinical, dialytic, and biological factors associated with poor HRQoL and poor KDQoL on univariate analysis are presented in Table 5.
| Factors | Poor HRQOL | Poor KDQOL | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-Value | OR | 95% CI | P-Value | |
| Anemia | ||||||
| Yes (Hgb < 11.0 g/dL) | 1.56 | 0.98 - 2.43 | 0.040 | 0.82 | 0.47 - 1.42 | 0.481 |
| No (Hgb ≥ 11.0 g/dL) | 1 | / | / | 1 | / | / |
| Respect hygieno-dietetic rules | ||||||
| Respect | 0.97 | 0.90 - 2.08 | 0.971 | 2.47 | 0.31 - 13.79 | 0.393 |
| No respect | 1 | / | / | |||
| Toxic habits | ||||||
| Yes | 2.75 | 0.80 - 9.47 | 0.108 | 0.70 | 0.26 - 1.86 | 0.486 |
| No | 1 | / | / | 1 | / | / |
| Body mass index (kg/m²) | ||||||
| < 18.5 | 1.78 | 0.94 - 3.40 | 0.076 | 1.28 | 0.63 - 2.57 | 0.483 |
| 18.5 - 24.9 | 1.52 | 0.96 - 2.41 | 0.072 | 1.31 | 0.78 - 2.19 | 0.300 |
| 25 - 29.9 | 1 | / | / | 1 | / | / |
| Hypertension | ||||||
| Yes | 0.821 | 0.54 - 1.24 | 0.367 | 0.63 | 0.39 - 1.00 | 0.053 |
| No | 1 | / | / | / | / | / |
| Diabetes mellitus | ||||||
| Yes | 0.75 | 0.49 - 1.15 | 0.199 | 0.83 | 0.52 - 1.32 | 0.433 |
| No | 1 | / | / | 1 | / | / |
| Cardiovascular diseases | ||||||
| Yes | 0.49 | 0.19 - 1.22 | 0.126 | 1.08 | 0.35 - 3.33 | 0.883 |
| No | 1 | / | / | 1 | / | / |
| Systemic diseases | ||||||
| Yes | 1.25 | 0.12 - 12.19 | 0.844 | 0.26 | 0.03 - 1.91 | 0.189 |
| No | 1 | / | / | 1 | / | / |
| Cancer | ||||||
| Yes | 0.41 | 0.05 - 2.97 | 0.381 | 0.41 | 0.05 - 2.97 | 0.381 |
| No | 1 | / | / | 1 | / | / |
| Liver diseases | ||||||
| Yes | 0.83 | 0.07 - 9.28 | 0.883 | 0.53 | 0.04 - 6.01 | 0.616 |
| No | 1 | / | / | 1 | / | / |
| Interdialytic weight gain (IDWG) | ||||||
| < 1 kg | 1.68 | 0.96 - 2.93 | 0.065 | 1.68 | 0.93 - 3.02 | 0.082 |
| 1 - 2 kg | 1.17 | 0.66 - 2.06 | 0.584 | 1.82 | 0.97 - 3.41 | 0.058 |
| > 2 kg | 1 | / | / | 1 | / | / |
| Duration on hemodialysis / mouths | ||||||
| < 50 | 1.67 | 0.96 - 2.90 | 0.068 | 1.71 | 0.95 - 3.08 | 0.072 |
| 50 - 100 | 1.17 | 0.66 - 2.06 | 0.583 | 1.78 | 0.95 - 3.33 | 0.062 |
| > 10 | 1 | / | / | 1 | / | / |
| Number of dialysis sessions / week | ||||||
| 2 Sessions | 2.14 | 1.12 - 4.08 | 0.021 | 1.76 | 0.87 - 3.53 | 0.113 |
| 3 Sessions | 1 | / | / | 1 | / | / |
| Vascular access | ||||||
| AVF proximal | 0.65 | 0.20 - 2.12 | 0.477 | 0. 25 | 0.03 - 1.99 | 0.191 |
| AVF distal | 0.76 | 0.24 - 2.14 | 0.649 | 0.21 | 0.02 - 1.61 | 0.134 |
| Tunneled jugular catheter | 1 | / | / | 1 | / | / |
| PTH (mg/mL) | ||||||
| < 300 | 0.90 | 0.50 - 1.61 | 0.729 | 1.46 | 0.70 - 3.03 | 0.309 |
| 300 - 600 | 1.59 | 1.00 - 2.51 | 0.644 | 0.96 | 0.59 - 1.57 | 0.901 |
| > 600 | 1 | / | / | 1 | / | / |
| Ca (mmol/L) | ||||||
| < 90 | 0.79 | 0.15 - 4.00 | 0.782 | 0.54 | 0.06 - 4.46 | 0.569 |
| 90 -105 | 0.77 | 0.13 - 4.41 | 0.777 | 0.37 | 0.04 - 3.42 | 0.386 |
| > 105 | 1 | / | / | 1 | / | / |
| P04 (mg/L) | ||||||
| < 25 | 0.54 | 0.20 - 1.49 | 0.260 | 0.79 | 0.15 - 4.00 | 0.782 |
| 25 - 45 | 1.39 | 0.91 - 2.12 | 0.325 | 0.77 | 0.13 - 4.41 | 0.777 |
| > 45 | 1 | / | / | 1 | / | / |
| Albumin (g/L) | ||||||
| < 38 | 0.74 | 0.41 - 1.32 | 0.313 | 0.77 | 0.40 - 1.50 | 0.458 |
| 38 - 50 | 0.99 | 0.57 - 1.72 | 0.986 | 0.83 | 0.45 - 1.55 | 0.579 |
| > 50 | 1 | / | / | / | / | / |
Abbreviations: OR, odds ratio; CI, confidence interval.
4.3.2. Multivariate Analysis
In univariate analysis, age, anemia, marital status, toxic habits, duration of hemodialysis, living conditions, interdialytic weight gain, frequency of dialysis sessions per week, comorbidities (diabetes and cardiovascular disease), and BMI were associated with poor HRQoL. Those associated with poor KDQoL were age, occupational status, support for medical costs, duration of hemodialysis, frequency of dialysis sessions per week, comorbidities (arterial hypertension), and vascular access.
These variables were chosen because of their correlation with the poor QoL in the two cores generic HRQoL and specific KDQoL, identified by univariate analysis and reported in the literature.
In multivariate analysis, factors that presented a strong statistical correlation (P < 0.05) with the poor level of QoL in HRQoL were: Anemia and frequency of dialysis sessions per week (Table 6), and for poor KDQoL, variables of age, professional status, and support for medical costs showed strong statistical correlation (Table 7).
| Factors | Poor HRQOL | ||
|---|---|---|---|
| OR | 95% CI | P-Value | |
| Anemia | |||
| Yes (Hgb < 11.0 g/dL) | 1.69 | 1.02 - 2.79 | 0.037 |
| Number of dialysis sessions / week | |||
| 2 Sessions | 2.24 | 1.04 - 4.66 | 0.030 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval.
| Factors | Poor KDQOL | ||
|---|---|---|---|
| OR | 95% CI | P-Value | |
| Age (y) | |||
| 46 - 65 | 0.45 | 0.24 - 0.79 | 0.006 |
| Occupational status | |||
| Employee | 6.15 | 1.48 - 8.53 | 0.028 |
| Support for medical costs | |||
| With support | 0.31 | 0.13 - 0.73 | 0.007 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval.
5. Discussion
The results of this study revealed that in the HRQoL, the mean score was 40.27 points. It is almost similar to the value reported by Zouari from Tunisia (ie, 38.2) (30). Moreover, this finding agrees with two series from Morocco and the United States, which, respectively, indicated that scores on these subscales were between 32.6 and 44.2 points in the study of Chrifi Alaoui (21), and between 36.6 and 49 points in the study of Cohen (31). While in Al Salmi's series from Oman (18) and in Tannor's work from South Africa (32), this rate was between 58.23 and 59.3 points, respectively. This difference may be due to the difference in age, as the mean age was 56 in our study, 49 in Zouari's study (30), and 61 in Cohen's study (31). While in Al Salmi's work (18) and Tannor's series (32), the mean age did not exceed 42, and the mean score HRQOL was higher. In the literature, the inverse association between QoL and age is explained by the deterioration of physical health and the decline in adaptive capacities (33).
In this study, the mean score of KDQoL was 40.74 points, which is consistent with a study conducted in Columbia (34), with a mean of 35.0 in the domain of the disease burden. However, the results of another study from Vietnam (35) and from South Africa (32) showed that scores on these subscales were, respectively, between 51.3 and 65 points. This difference can be justified by the difference in the QoL assessment context and health care systems (33, 34).
In our study, 29.5% of participants had a poor HRQOL, and 21.3% had poor KDQoL. Several factors were associated with poor HRQoL, such as anemia and the frequency of dialysis sessions per week. People with anemia are more likely to have a lower HRQoL than people without anemia; this finding is not consistent with some studies that reported no association between hemoglobin and QoL (31). However, another study demonstrated that high hemoglobin level is significantly associated with better HRQoL (19) and reported that anemia commonly contributes to poor QoL in patients with CKD. From a clinical perspective, it makes sense that anemia affects HRQoL in hemodialysis patients, as it is a frequent comorbidity of CKD and is associated with an elevated risk of CKD progression, cardiovascular problems, and mortality (36).
Therefore, anemia remains a significant problem in patients with CKD. Hence, there is a need for new therapeutic approaches (32).
The frequency of dialysis sessions per week is the second factor that indicated an association with poor HRQoL; retained in multivariate analysis, patients who receive two dialysis sessions per week are more likely to have poor HRQoL in comparison to those who receive three dialysis sessions per week. This can be explained by the fact that shortening the interdialytic intervals can reduce the instantaneous ultrafiltration flow rate and allows an improvement in the hemodynamic tolerance of the sessions. In addition, it alleviates post-dialytic tiredness and results in better sleep adequacy (37).
On the one hand, poor HRQoL may be due to the iatrogenic effects of the usual rhythm of hemodialysis, which is insufficient to control fluid and solute levels (15). On the other hand, hemodialysis patients may have many troublesome symptoms of the uremic syndrome related to the persistence of protein-bound uremic toxins and small peptides (named middle molecules), which are not effectively removed by current dialysis modalities (13). Thus, intensive hemodialysis can directly contribute to decreasing iatrogenic effects and thereby improving HRQoL (15) and the development of dialysis methods that can improve the removal of all these middle molecules. It is a promising approach to achieving better outcomes and, thereby, a better QoL for hemodialysis patients (13). In this study, the number of dialyses was retained in the univariate analysis as a factor associated with the alteration of the QoL (30). On the other hand, in another series (38), extending weekly hemodialysis hours was not associated with poor QoL. Nevertheless, new approaches and modalities for dialysis that are cost-effective, accessible, and provide better outcomes for patients must be designed as a matter of urgency (13) to improve their QoL.
Our findings show that 21.3% of participants had poor KDQoL. In addition, the following variables were associated with poor KDQoL: age, professional status, and support for medical costs. The age between 45 and 65 years is considered a protective factor (CI: 0.24 - 0.89, P = 0.006) against a poor KDQoL, insofar as patients who are part of this age are not likely to have poor KDQoL compared to others who are over 65 years old. A similar association is seen in previous studies (30, 38), the results of which indicated an association between age and poor QoL. Also, patients of this age (ie, 46 - 65 years) have a better QoL in comparison to their younger counterparts. This may be due to a better adaptation to chronic diseases and lower expectations of older patients compared to younger ones (18, 24).
Regarding occupational status, the results of our study suggest that professionally active patients have lower QoL scores than patients who do not work, which is also reported by a recent Saudi study that found higher QoL scores among those who did not work and stayed at home (39). However, our findings are contrary to the results reported by another series (40), which say that professionally active patients had a higher QoL scores. Our finding may be justified by the fact that work increases fatigue in hemodialysis patients, which in turn affects their daily life, physical activities, and well-being. Fatigue is known to be one of the most reported symptoms by hemodialysis patients that negatively and profoundly affects their QoL (41).
For the support of medical costs, it is considered as a protective factor (CI: 0.13 - 0.73, P = 0.007) against poor KDQoL; that is, patients who have no support for medical costs are more likely to have a lower KDQoL, as shown by two recent studies in Nepal and Ethiopia (38, 42), which reported that patients who received ESRD paid less medical costs, and those who were able to pay all medical costs had a better QoL compared to patients with no support for medical costs. In fact, the main reason for the economic burden on CKD patients is medical costs (the cost of drugs and surgery/operations) (35). Nonetheless, patients who receive medical cost support and pay fewer medical costs are more likely to access medical services and maintain their health status, which may reduce health problems and comorbidities, and it is known to impact survival, hospitalization, and HRQoL (24).
Comorbidity was also retained in the univariate analysis as a risk factor associated with a low level of QoL, in particular diabetes and cardiovascular diseases, which are associated with poor HRQoL, and arterial hypertension, which was associated with poor KDQoL. However, in multivariate analysis, the comorbidity had no correlation with a low level of QoL either in HRQoL or in KDQoL. Several studies (18, 42, 43) reported that patients with underlying health problems had poor HRQoL and KDQoL, as well as patients with ESRD and a history of cardiovascular disease who had a poor HRQoL (44).
According to the results of our study, the residence origin and the education level have no statistical correlation with the low level of QoL, either for HRQoL or KDQoL, unlike other studies that reported higher education level was associated with better KDQoL and people from urban areas had better HRQoL and slightly better KDQoL than those from rural areas.
Based on the findings, there was no association between albumin level and QoL, which is not in line with other studies that reported higher albumin levels were associated with better HRQoL (42, 43).
In our study, both medical and non-medical factors were associated with poor HRQoL and KDQoL. Indeed, according to our results: sociodemographic factors (ie, occupational status, age, and support for medical costs) and clinico-dialytic factors (ie, anemia and number of dialysis sessions per week) were significantly associated with HRQoL and KDQoL. Therefore, effective interventions are needed to address both medical and non-medical parameters affecting the QoL of hemodialysis patients.
5.1. Limitations
Our study had some limitations. Firstly, the study was conducted in a single geographic region and concerns a single population of hemodialysis patients, which does not allow generalization to other hemodialysis populations. Secondly, the study was performed following a cross-sectional design, which its results only show the association of factors with HRQoL and KDQoL rather than causal inference.
5.2. Conclusions
Patients with anemia who had a lower number of dialysis sessions per week had a poorer HRQoL. Also, patients aged between 45 and 65 years who had support for medical costs and did not work had a better KDQoL. In this sense, the government and decision-makers should be aware of the interest in improving the socioeconomic level of patients, particularly for hemodialysis patients, reducing the cost of drugs, or ensuring appropriate health insurance for them that would allow broad and affordable access to medical services. Effective interventions are needed to actively treat anemic and intensify dialysis sessions or even develop cost-effective, efficient, and accessible dialysis methods and modalities that can decrease the burden of symptoms and ensure patients' better quality of life. Therefore, a comprehensive and multidisciplinary approach is needed to address all aspects that can improve the quality of life of hemodialysis patients in the Souss Massa region, involving the government, the Ministry of Health, nephrologists, nurses, psychologists, pharmacists, social workers, and families.