1. Background
Stress urinary incontinence (SUI), defines as "urine leakage upon effort," is the most common type of incontinence and etiologically a multifactorial problem. Its pathophysiology includes impairment in the mechanisms maintaining urinary continence from anatomical to functional and neurophysiological problems (1). The pelvic floor muscles (PFMs) are an essential component responsible for urinary incontinence (UI). These muscles are involved in activities that increase intra-abdominal pressure. The PFMs are often made up of type I slow twitch fibers, which produce resting tone and constant contraction (2, 3) that keeps the pelvic viscera in place (4). They also have other type II fast-twitch fibers (2, 3), which intervene rapidly to interact with the activities that increase intra-abdominal pressure (5).
Nervous system disorders may lead to SUI, but the neural circuitry related to the total urethral closing mechanism is not yet fully understood (1). PFMs and their innervation play a significant role in the urethral closure. Proprioception mediated by both central and peripheral mechanisms has not yet been measured in the SUI population (6). Proprioception comprises the sense of movement, force, effort, and balance in the musculoskeletal system, which can be affected by neural circuitry dysfunction (7). Force sense (FS) is generated by central and/or peripheral mechanisms (8). The central mechanism happens through the corollary discharge that arises from the motor cortex, and the peripheral mechanism is followed by the information sent by the mechanoreceptors, especially the Golgi tendon, to the central system (8).
Understanding the amount of contractile force produced in the muscle during contraction is essential to muscle function (9). In the case of SUI women, where PFMs are weaker and damaged because of elongation or laxity (10), muscle receptors are desensitized, reducing proprioception (11). Proprioceptive dysfunction, in turn, can affect the timing and pre-contraction of PFMs (12). During coughing, urethral pressure increases typically 100 to 300 msec before intravesical pressure rises. In SUI, however, this urethral pressure rise is delayed due to a lack of PFM pre-contraction (13). Similar observations have been reported during postural activities that increase intra-abdominal pressure (14). In balance assessment, SUI women also had higher EMG of the trunk muscles and PFMs compared to continent controls, causing trunk stiffness and reducing proprioception accuracy (15).
Previous studies remarked on the importance of proprioception in motor control and its alterations in women with SUI (6). Also, an animal study showed atypical expression of pelvic proprioceptors in rats with SUI, affecting PFM contractibility (16).
Studies have mainly concentrated on the joint sense of position to investigate proprioception, and there is a finite amount of evidence on force sense (17). Research regarding FS accuracy in women with SUI is also sparse.
2. Objectives
The primary aim of this study was to compare the accuracy of PFM FS in women with and without SUI. Second, we aimed to evaluate the accuracy of FS between various lengths and tensions of PFMs in these two groups.
3. Methods
3.1. Settings and Participants
This case-control study was conducted in the pelvic floor clinic of the School of Rehabilitation, Iran University of Medical Sciences, between June and August 2019. Inclusion criteria included: (a) non-pregnant women with and without SUI who had given birth at least six months ago; (b) a score of two or more on the Oxford scale (18) because the dynamometer could not detect very low forces; and (c) SUI diagnosis by a urologist.
Exclusion criteria included: (a) women with stage 3 or higher prolapse (Pop Q > 2) (19); (b) previous physiotherapy treatment for SUI; (c) vaginal ulcers or infections preventing device introduction into the vagina, (d) skeletal muscle disorders preventing PFM strength measurements, and (e) any drug consumption affecting muscle strength. Athlete women were also excluded from the study. Signed informed consent was obtained before entering the study. The Ethics Committee of Iran University of Medical Sciences approved the study protocol (IR.IUMS.REC.1397.200).
3.2. Dynamometric Evaluation
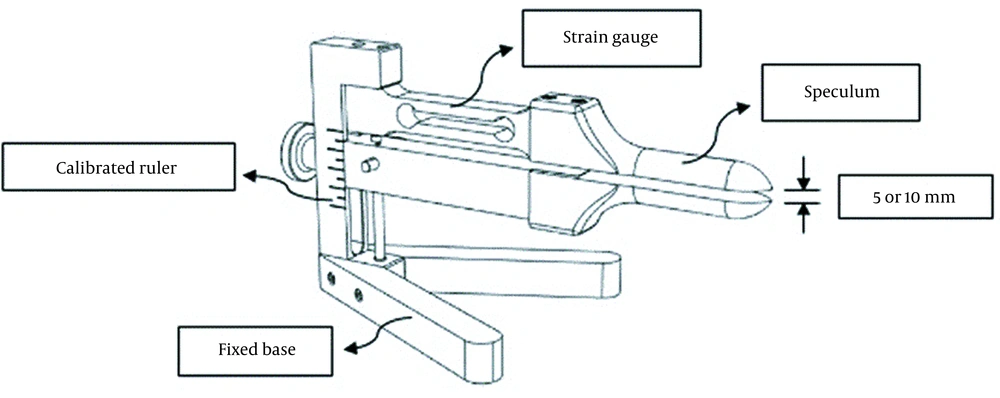
For measuring force reproduction, we developed a dynamometer whose hardware was the same as the dynamometer designed by Dumoulin et al. (20). This device consisted of a speculum (fixed upper arm and a movable lower arm with an adjusting screw to change the vaginal opening) with thicknesses of 6 mm and 8 mm for the upper and lower arms, respectively, a calibrated ruler to accurately determine the 5 and 10 mm openings that corresponded to vaginal apertures of 19 and 24 mm when the aforementioned thicknesses of the upper and lower arms were added to these openings (21), a fixed base to prevent errors of movement, and a strain gauge connected to the software (Figure 1). Dynamometer openings in this study were selected based on the previous research by Dumoulin et al., which reported better reliability in the opening of 10 mm and lower reliability in the larger diameter because of the patient's discomfort (21). Also, the dynamometer opening affects the reliability of pelvic floor measurements (21). Moreover, according to prior studies, muscle length affects muscle force (9), so we also measured the minimal opening of 5 mm.
The software showed the amount of contraction force in Newton. The initial phase was continuous biofeedback through the dynamometer software to teach the person correct PFM contraction and appropriate device placement. Second, maximum voluntary contraction (MVC) was recorded, followed by the FS, which showed the percentage of MVC. As previous studies demonstrated the key role of muscle fiber type in motor control (22, 23), in this study, we indirectly evaluated type I and II muscle fibers by generating 40% and 70% of muscle contraction, respectively (24). The available dynamometers did not have software to define different percentages of MVC and lacked the capability of determining the threshold and giving visual feedback to the patient prior to the FS test.
3.3. Procedure
After information and instruction, the patient was asked to empty her bladder before the test. The patient was in a crook lying position during the test. To facilitate correct PFM contraction without the dominant activity of synergistic muscles (abdominal, adductor, and glutei muscles), first, vaginal palpation was done as a reinforcement technique to educate the patient to contract PFMs specifically (25), and then, dynamometric evaluation was performed. The openings of 5-mm or 10-mm diameter were counterbalanced based on a randomization table. Before the speculum was placed intravaginally, its arms were covered with condoms and impregnated with a lubricant gel. The speculum was then inserted about 5 cm intravaginally between the PFMs and about 3.5 cm inside the vaginal hymen. If preferred, the patient was allowed to insert the speculum intravaginally herself.
Initially, to ensure comfort and correct device placement, inspect the muscle contraction diagram on the device monitor, and familiarize the patient with the test condition, three PFM contractions were performed. Then, MVCs were executed while watching the monitor raise the chart. Three MVCs with 60 seconds of rest between two consecutive MVCs were performed, and the highest value was recorded. Finally, force reproduction of 40% or 70% of MVC was tested as an FS. At this point, the MVC 40% or 70% threshold was set on the monitor, and we asked the participant to reproduce that force. The participant repeated the contraction 10 times for each MVC percentage, with five seconds of rest after each contraction. The monitor was then replaced far from the participant and instructed to produce six times the same force without visual feedback. Because it could have influenced the intra-abdominal pressure and changed PFM contractions, no verbal comments from the participants were allowed, and patients were asked not to hold their breath and continue their normal breathing. Instead, the assessor observed the contraction on the monitor, and the amount of force reproduction was recorded. All measurements were taken by a single investigator with five years of pelvic floor rehabilitation experience.
Force reproduction was recorded six times in both groups for 5- and 10-mm openings and 40% and 70% MVC. There was a five-second rest between repetitions and a five-minute rest between different assessment conditions. During the session, all assessments were performed by the same physical therapist.
The reliability of the dynamometer was reported in the study by Dumoulin et al. (21). Regarding the intra-tester reliability, six trials of force reproduction were taken at each of two different openings for both 40% and 70% MVC.
3.4. Statistical Analysis
Independent variables were group (without SUI versus with SUI), MVC% (40% versus 70%), and diameter of the speculum opening (5 mm versus 10 mm).
The Shapiro-Wilk test was used to evaluate the normal distribution of data. The independent t-test was used to compare baseline characteristics between groups for normally distributed variables. Wilcoxon signed-rank test was used to compare the number of deliveries at baseline between the groups.
To measure the accuracy of force reproduction, the difference between the value of the target force and the amount of force reproduced by individuals was used to measure the force reproduction error. As participants either overestimated or underestimated when reproducing the target force, the absolute value of this difference was used as a deviation from the target.
A one-way agreement model of intraclass correlation coefficient (ICC) was used to measure test-retest reliability within six repeats of the accuracy of force reproduction measures overall, as well as for each subgroup.
A linear mixed model regression was used to estimate the accuracy of force reproduction (dependent variable) between groups, diameters, and MVC%. The subject ID was used as a random effect to control the variability between subjects and the variability of six observations within each subject.
4. Results
A total of 41 women, 18 without SUI and 23 with SUI (mean ages 46.7 (SD 3.04) and 51.5 (1.86) years, respectively) were included (26, 27). The demographic characteristics of the participants are shown in Table 1. At baseline, the independent sample t-test showed no statistically significant difference between the two groups in age (P-value = 0.168) and BMI (P-value = 0.188). Wilcoxon signed-rank test showed no difference between the groups in the number of deliveries (P-value = 0.282). Since gestation, regardless of the mode of delivery, could affect PFMs (28), both cesarean and natural deliveries were included in this study.
| Healthy (N = 18) | Patients (N = 23) | |
|---|---|---|
| Age (y) | 46.7 ± 3.04 | 51.5 ± 1.86 |
| BMI (kg/m2) | 26.9 ± 0.93 | 30.6 ± 2.47 |
| Number of deliveries | 2.2 ± 0.42 | 2.82 ± 0.25 |
| MVC (5 mm) | 6.89 ± 3.5 | 3.54 ± 3.08 |
| MVC (10 mm) | 8.73 ± 4.11 | 4.81 ± 4.67 |
a Values are expressed as mean ± SD.
The ICC ranged from 0.677 to 0.951. The highest reliability was attributed to women with SUI-40%-5mm and women with SUI-70%-10mm, and the lowest was attributed to women without SUI-70%-10mm (Table 2).
| Groups | MVC% | Diameter | ICC | 95% CI |
|---|---|---|---|---|
| Healthy | 40% | 5 | 0.913 | 0.827 - 0.968 |
| 10 | 0.835 | 0.694 - 0.936 | ||
| 70% | 5 | 0.794 | 0.631 - 0.919 | |
| 10 | 0.677 | 0.469 - 0.862 | ||
| Patients | 40% | 5 | 0.951 | 0.912 - 0.977 |
| 10 | 0.943 | 0.899 - 0.973 | ||
| 70% | 5 | 0.842 | 0.735 - 0.923 | |
| 10 | 0.945 | 0.903 - 0.974 |
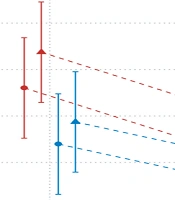
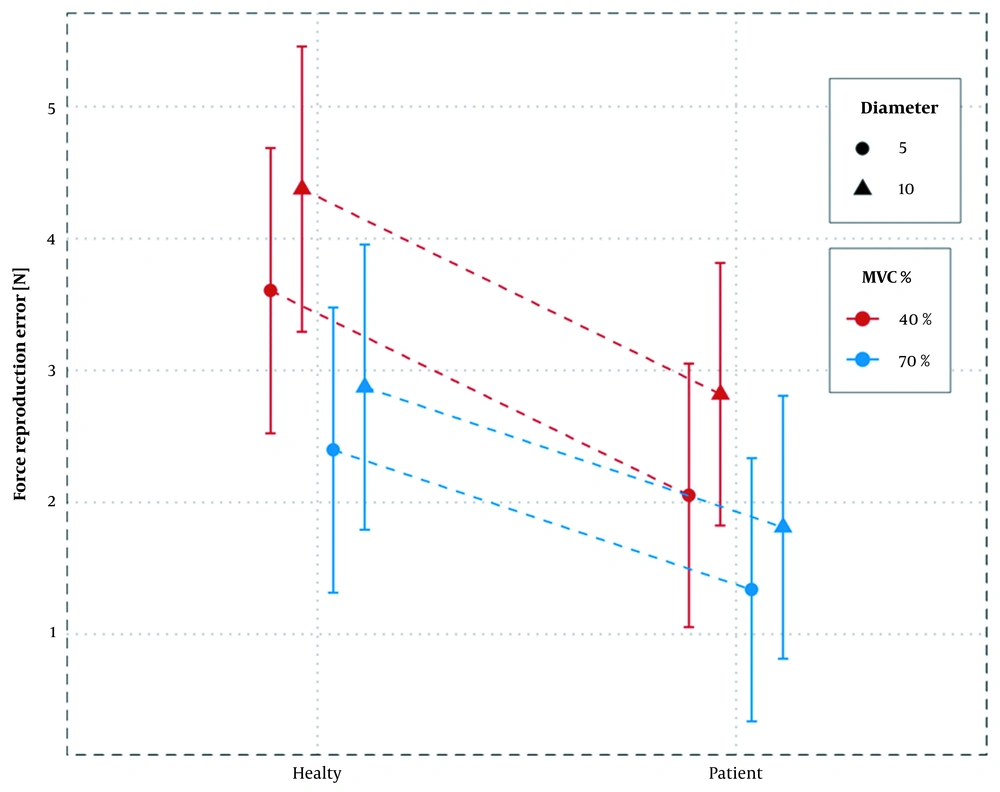
The accuracy of force reproduction differed between the groups (main effect: F (37) = 4.602, P-value = 0.038). In all test conditions, SUI women had higher force reproduction accuracy. Similarly, the force reproduction accuracy differed in terms of MVC percentage and opening diameter (main effect: f (808) = 50.094, P-value= 0.000 and f (808) = 19.417, P-value= 0.000, respectively). The lowest error estimate was found in SUI women reproducing 70% MVC in 5 mm speculum diameter (1.34 N), and the highest error was found in women without SUI in 40% MVC and 10 mm diameter (4.38 N), as presented in Table 3. A statistically significant interaction was observed between grouping and MVC percentage (F (808) = 6.852, P-value= 0.009). Besides, there was no significant association between MVC percentage and diameter (F (808) = 2.479, P-value = 0.115) and between diameter and group (F (808) = 0.0002, P-value = 0.986), as presented in Table 4.
| Groups | MVC% | Diameter | Mean | SE | df | 95% CI |
|---|---|---|---|---|---|---|
| Healthy | 40% | 5 | 3.60 | 0.53 | 38 | 2.52 - 4.69 |
| 10 | 4.38 | 0.53 | 38 | 3.29 - 5.46 | ||
| 70% | 5 | 2.40 | 0.53 | 38 | 1.31 - 3.48 | |
| 10 | 2.87 | 0.53 | 38 | 1.79 - 3.96 | ||
| Patients | 40% | 5 | 2.05 | 0.49 | 37 | 1.05 - 3.05 |
| 10 | 2.82 | 0.49 | 37 | 1.82 - 3.82 | ||
| 70% | 5 | 1.34 | 0.49 | 37 | 0.34 - 2.34 | |
| 10 | 1.81 | 0.49 | 37 | 0.81 - 2.81 |
Abbreviations: MVC, maximum voluntary contraction; mm, millimetres; SE, standard error of mean, df, degree of freedom; CI, confidence interval.
| Predictor Variable | Numerator df | Denumerator df | F-Value | P-Value |
|---|---|---|---|---|
| Group | 1 | 37 | 4.60 | 0.039 |
| MVC% | 1 | 808 | 50.09 | 0.000 |
| Diameter (mm) | 1 | 808 | 19.42 | 0.000 |
| Group × MVC% | 1 | 808 | 6.85 | 0.009 |
| Diameter × MVC% | 1 | 808 | 2.48 | 0.116 |
| Diameter × Group | 1 | 808 | 0.00 | 0.986 |
Abbreviations: MVC, maximum voluntary contraction; mm, millimetres; df, degree of freedom.
Women without SUI had higher error values in 40% MVC compared to 70% MVC (mean difference for both diameters = 1.35 N, P-value < 0.0001); besides, values were higher compared to women with SUI with a smaller difference (mean difference for both diameters = 0.86 N, P-value < 0.0001) (Figure 2). In terms of diameter, there was always a higher error in the 10 mm diameter of the applicator compared to 5 mm, irrespective of the group and MVC percentage. The mean difference of error was almost the same. Regarding MVC, the percentage error of force reproduction was always higher at 40% compared to 70%, irrespective of group and diameter, indicating no statistically significant association (Table 5).
| Diameter (mm) | MVC% | Group | Contrast | Mean Difference | SE | df | t-Ratio | P-Value |
|---|---|---|---|---|---|---|---|---|
| 5 | 40% | H - P | 1.55 | 0.72 | 37 | 2.14 | 0.039 | |
| 10 | 40% | H - P | 1.56 | 0.72 | 37 | 2.15 | 0.038 | |
| 5 | 70% | H - P | 1.06 | 0.72 | 37 | 1.46 | 0.152 | |
| 10 | 70% | H - P | 1.06 | 0.72 | 37 | 1.47 | 0.150 | |
| 40% | H | 10 - 5 | 0.77 | 0.17 | 808 | 4.41 | 0.000 | |
| 40% | P | 10 - 5 | 0.77 | 0.15 | 808 | 4.95 | 0.000 | |
| 70% | H | 10 - 5 | 0.48 | 0.17 | 808 | 2.73 | 0.006 | |
| 70% | P | 10 - 5 | 0.47 | 0.15 | 808 | 3.06 | 0.002 | |
| 5 | H | 40% - 70% | 1.21 | 0.17 | 808 | 7.08 | < 0.001 | |
| 5 | P | 40% - 70% | 0.71 | 0.15 | 808 | 4.61 | < 0.001 | |
| 10 | H | 40% - 70% | 1.50 | 0.17 | 808 | 8.79 | < 0.001 | |
| 10 | P | 40% - 70% | 1.01 | 0.15 | 808 | 6.62 | < 0.001 |
Abbreviations: MVC, maximum voluntary contraction; mm, millimetres; SE, standard error of mean; df, degree of freedom; H, healthy; P, patients.
5. Discussion
Inter- and intragroup analyses were performed to examine the accuracy of FS in women with and without SUI; muscle length and tension were also examined. In general, the force reproduction error was more in women without SUI than in those with SUI.
The highest error was found in women without SUI at 40% MVC-10 mm and the lowest one in SUI women at 70% MVC-5 mm. With an increase in speculum diameter in both groups, the error rate also increased, irrespective of the MVC percentage. In fact, increasing the length of muscle fibers led to an increase in force reproduction error. Also, due to the reduction in force reproduction accuracy during application of 40% compared to 70% in both groups, the accuracy of force reproduction was lower during the action of slow-twitch fibers, which exceeded the number of fast-twitch fibers in PFMs. In both groups, the force reproduction error was higher in 40% MVC-10 mm than in other conditions, possibly indicating a general pattern in the accuracy of PFM force reproduction.
In this study, the amount of force reproduction error in both groups was lower in 70% MVC than in 40%, which is probably due to the activity of both slow and fast-twitch fibers to enhance a stronger contraction in reproducing 70% MVC compared to 40% in which the slow-twitch fibers are mostly activated (24). Slow-twitch fibers are more designed for endurance activities like maintaining posture. Some studies investigated the postural activity of PFMs in women with SUI and reported postural control alterations, which is consistent with the results of this study (14, 29). Smith et al. (14, 29) evaluated PFMs and abdominal muscles by electromyography during perturbation. Their results showed that although PFM in women with SUI was delayed in response to perturbation and pre-contraction did not occur in these people, PFM and abdominal muscles were more active during a postural perturbation compared to women without SUI. We consider this a motor control strategy to avoid urine leakage and compensation for the delayed onset of PFMs. Another study by Smith et al. examined the balance in women with SUI in quiet standing (during quiet standing, humans invariably sway to maintain balance, and this motion is measured using the anterior-posterior and the medial-lateral components of the net center of pressure (COP)). In this study, the electromyographic activity of PFM and trunk muscles, as well as COP displacement, were higher in SUI women than in women without SUI, which probably decreased the proprioception acuity following higher muscle activity (15). Studies have shown that systemic noise increases due to increased fusimotor activity, which can reduce the sensitivity of the muscle spindle and impair balance in these individuals (30). On the other hand, increasing the cognitive attention of these people to continence may increase the body sway (31). In another study that assessed the postural control in patients with SUI, controlling posture while the bladder is full was impaired compared to those in the control group. It could be due to systemic noise regarding the proven role of the periaqueductal gray in both micturition and controlling body activity. Hence, bladder fullness affected this system and altered postural control in women with SUI (32).
Force perception depends on central and peripheral mechanisms. In this study, we addressed the peripheral components. In general, in all conditions, the amount of reproduction error, contrary to the hypothesis of this study, was higher in women without SUI than in SUI women. The question is whether any central components should be addressed in SUI women. This can be explained through automaticity, which reduces the need to pay more attention to tasks and cognitive control in women without SUI than in those with SUI. Shiffrin and Schneider (33) stated that the automatic process is difficult to inhibit, correct, and ignore when learning occurs. This is because stimulus-response maps are stored in the brain permanently, resulting in a stimulation followed by an automatic answer. This process disrupts the ability to create variable responses while improving performance (33). Therefore, a lack of attention and cognitive resource related to automatic behaviors makes it harder to control automatic responses than controlled tasks. Evidence has also shown that motor performance is directly affected by the performer's focus and attention. Focusing on the effect of movement, known as the external focus of attention, creates better motor performance than focusing only on the pattern of a movement, known as the internal focus of attention. According to the constrained action hypothesis, external focus facilitates motor performance by improving automatic movement control. In contrast, the internal focus of attention causes conscious control of movement and disrupts the normal automatic process (34). Studies have also shown that performing an activity consciously leads to more EMG activity than automatic performance because automatic performance is more efficient in motor control (35).
Functional MRI studies have shown that in SUI patients having physical therapy, the activity of the primary motor and somatosensory areas increases gradually while reducing the activity of the premotor and supplementary motor areas (36). These findings indicate more efficient PFM activity and less attentional demand (37).
In our study, people with constant attention to the pelvic floor area and thinking to prevent UI probably switched their control from automatic to conscious ones; therefore, their attention to the pelvic floor was increased, reproducing the force more carefully. Gilpin et al., who compared the EMG activity of PFMs in SUI women and those with continence, found that motor unit fiber density was higher in SUI than in continent women, which could be due to subsequent muscle reinnervation. The biopsy study showed that the diameter of type I muscle fibers was significantly larger in the peri-urethral area of SUI women than in continent women (2), possibly due to participants' constant attention to the area and trying to maintain muscle contraction to avoid UI.
In this study, both groups had less error in 5 mm compared to 10 mm. There is a possibility that patients felt more uncomfortable with the larger opening of the speculum, which, in turn, inhibited muscles and impaired their function.
According to some investigations on SUI women's balance and postural activity (14, 15), PFM activity is increased when balance and postural control are challenged. Also, the results of this study showed that the force reproduction accuracy was more in SUI women than in those with continence. Regarding these findings, the automatic activity of PFM in SUI women may be impaired, and they might constantly pay attention to this area and contract their muscles to avoid UI.
In this study, the presence of a dynamometer speculum with increasing sensory input may be associated with the increasing attention of participants, which might have reduced their force reproduction error. Therefore, assessing force reproduction accuracy without placing a device in the vagina could be the subject of further investigations.
Based on our knowledge, this study is the first to evaluate the accuracy of FS as an indicator of proprioception in the PFMs in SUI women. However, there were some limitations in this study. One of them was the relatively small sample size due to the limited sources, time limitation, and patient refusal to expose their perineal area and insert the dynamometer. Another limitation was the lack of pain and discomfort measurement in patients while placing the speculum in the vagina. Previous studies have also shown that nerve damage frequently occurs during childbirth and is a common finding in SUI women, so it might be better to check nerve supply and muscle fibers in this area before examining the function of PFM. One of the inevitable limitations of this study was the utilization of identical dynamometer openings in all women with different vaginal apertures and PFMs lengths. In this study, only women with a score of two or more on the Oxford Scale were included, and given that many SUI women may have lower muscle strength, it is recommended that future studies also assess FS in women with weaker PFMs. Due to the limited number of patients who consented to participate in this study, we included all types of SUI without defining a cutoff for severity. It is recommended that future studies subcategorize different types of SUI in different populations, including pregnant, postpartum, and post-menopausal women. Finally, since the lumbopelvic posture could affect PFMs (38), it is recommended that future studies homogenize both groups in this respect.
5.1. Conclusions
This study evaluated the sense of force reproduction accuracy in women with and without SUI. The SUI women were more accurate in force reproduction. It is possible that in SUI women, automaticity might have been decreased in PFMs. Therefore, they paid more attention to this area and tried to maintain its contraction during activities. A potential goal in treating these women could be restoring the automatic activity in PFMs, so involuntary contractions of the PFM should also be assessed.