1. Background
The capacity to perform jumps is crucial for achieving success in numerous sports, including volleyball, diving, and American football, as it enables athletes to compete at higher levels. Jumping ability plays a significant role in these sports and serves as an indicator of lower extremity strength (1, 2). The countermovement jump (CMJ) is a vertical jump variation that involves both an eccentric and concentric phase, resulting in optimal muscle contraction (3). Over the years, the CMJ has been extensively utilized to evaluate the effectiveness of different training modalities. It is widely recognized as one of the most commonly employed tests for monitoring neuromuscular status in both individual and team sports (4-6).
The CMJ assessment offers a range of kinetics and kinematics variables that can be effectively incorporated into training programs to enhance athletes' performance (7). Notably, the rate of force development (RFD), which is influenced by motor unit activation, has a strong correlation with an athlete's explosive power production and CMJ performance (8). It is reasonable to assume that improving the biomechanical parameters of the CMJ, such as RFD, could potentially lead to enhancements in CMJ performance.
In recent decades, there has been an increasing emphasis on understanding the role of the brain in performance limitations and enhancements. Extensive research has demonstrated the significant influence of the brain on physical performance (9, 10). The central nervous system has the capacity to augment muscle strength through increased recruitment of motor units. Consequently, alterations in cortical or corticospinal excitability can contribute to improved muscle strength (11). In light of these findings, transcranial direct current stimulation (tDCS) has emerged as a promising method for enhancing athletic performance (12).
As a non-invasive, user-friendly, safe, and painless brain stimulation technique, tDCS has the ability to modulate the excitability of brain pathways and induce cortical plasticity (13, 14). By applying a weak direct current (up to 2 mA for 3 - 20 minutes) to the scalp, tDCS can effectively regulate cortical excitability. This technique has the potential to enhance performance by increasing corticospinal excitability or optimizing muscle recruitment strategies (15, 16). Furthermore, tDCS has been shown to improve motor learning and contribute to enhanced performance (17).
Although the precise mechanisms underlying muscle strength are not yet fully comprehended, several factors such as age, gender, neuromuscular and muscle characteristics, and fatigue are believed to contribute to this process. Additionally, increased activation of agonist muscles and motor unit firing rate appear to influence power generation. In line with this, tDCS has the potential to enhance strength-related performances, such as the CMJ, by modulating cortical or corticospinal tract excitability (18).
There is a growing interest in utilizing tDCS as a method to enhance athletic performance through modulation of the motor cortex excitability. Numerous studies have investigated the impact of tDCS on muscle strength in healthy individuals (19, 20). While some studies have reported an increase in strength with tDCS application (20, 21), other studies have not found a significant relationship between tDCS and enhanced muscle strength (22, 23). By examining the effects of tDCS on the kinetic variables of the CMJ, we can gain insights into the influence of tDCS on movement strategies (24). Given the pivotal role of kinetic components in jumping and the divergent findings of previous research (20-23), we have undertaken this study.
To date, no study has specifically investigated the impact of tDCS on the kinetic variables of jumping, which is a fundamental exercise in numerous sports. In order to establish a link between potential kinetic improvements and tDCS, we included a control group that underwent the same procedure but without any stimulation.
2. Objectives
The objective of this investigation was to assess the effects of anodal tDCS (a-tDCS) on the kinetic variables of the CMJ and compare them to both the Sham tDCS and non-intervention groups.
3. Methods
3.1. Subjects
We conducted a power analysis (G*Power v 3.1) with a critical P-value of 0.05 and a power of 0.95, based on a similar study (25), which yielded a sample size of 48 subjects. Forty-eight young, healthy individuals (mean ± standard deviations (SD); age: 22.12 ± 2.11 years; body mass: 65.66 ± 14.41 kg; height: 168.31 ± 11.18 cm), classified as non-elite athletes in jumping sports like handball, volleyball, and basketball (up to 3 hours of training per week), volunteered to participate in this study. Participants' anthropometric measurements are displayed in Table 1. Participants were excluded if they had orthopedic, cardiovascular, or neurological diseases. Those who took neuropsychiatric drugs and caffeinated beverages on the experiment day were also excluded, as well as those who had severe migraines or a metal plate in the head. Before starting the study, all participants signed an informed consent form. The experiment was approved by the institutional ethics committee of Semnan University of Medical Sciences (IR.SEMUMS.REC.1399.200).
3.2. General Experimental Design
A double-blinded, randomized, controlled design was used to investigate the effects of tDCS on the kinetic parameters of the CMJ. Participants underwent three experimental conditions: Anodal tDCS, sham tDCS, and control (non-intervention). In the anodal tDCS and sham tDCS groups, the kinetic assessment of the lower limb during CMJ was conducted using a force plate before and after three intervention sessions, which were spaced 48 hours apart. One week after the last intervention session, an evaluation session was conducted for these two groups to assess the persistence of stimulation effects. In the control group, the kinetic assessment of the lower limbs during CMJ was evaluated, with a time interval equivalent to that of the other groups.
3.3. Procedure
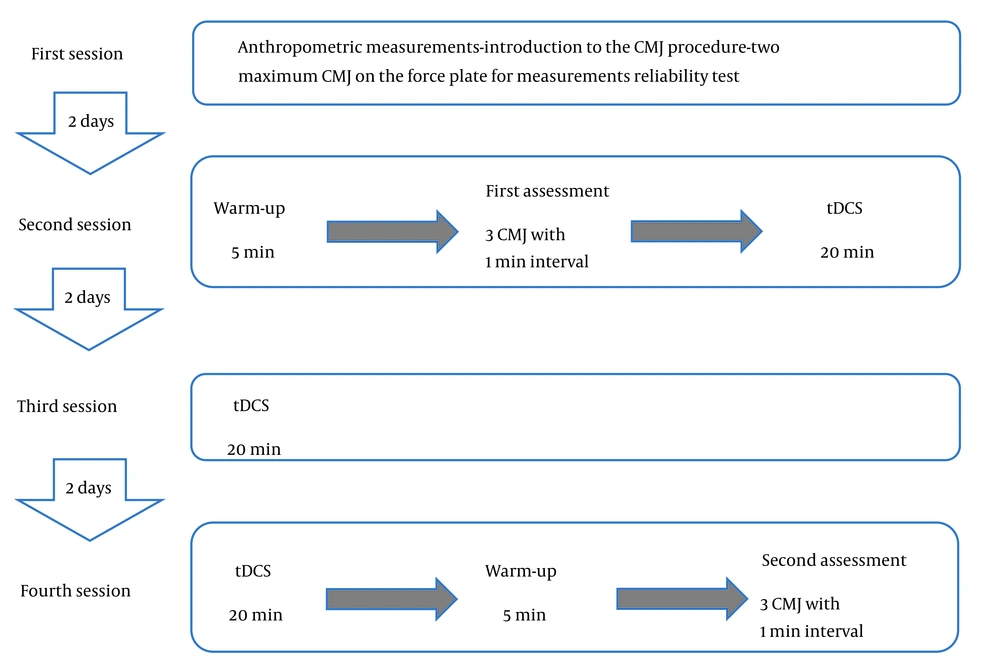
Participants visited the laboratory on four occasions. During the initial session, participants underwent anthropometric measurements and were familiarized with the CMJ procedure, as well as the reliability test for CMJ kinetic measurements. Following a five-minute warm-up, the second session involved participants performing three jumps with a one-minute rest interval between each jump to determine their optimal jump performance.
After a period of recovery, the participants were seated on a laboratory chair for the administration of tDCS in the Anodal tDCS group. An electrical current with an intensity of 2 mA was delivered for 20 minutes using a pair of saline-soaked surface electrodes (5 × 7 cm) (26) connected to a continuous current stimulation device (Activa DOS2, activatech, USA). The anode was positioned over the Cz electrode region following the 10 - 20 system (27), while the cathodal electrode was placed over the right orbitofrontal cortex. In the sham tDCS group, the setup mirrored that of the anodal tDCS group, except the current ceased after 30 seconds. Any adverse effects of tDCS were assessed based on participants' reports of discomfort or unusual sensations. During the third session (48 hours after the second session), tDCS was administered in a manner similar to the second session. In the fourth session (48 hours after the third session), tDCS was administered in the same way as the second session, followed by the completion of three CMJs, as depicted in Figure 1. The control group (non-intervention) solely took part in the evaluations without any intervention. Factors such as time of day, sleep, and nutritional status were standardized across all groups. The evaluator responsible for assessing the CMJ was blind to the stimulation conditions.
3.4. Countermovement Jump Performance
To execute the CMJ, the athlete initiates from a standing position. The jump commences with an upward acceleration originating from below the center of gravity, with the knees flexed approximately 90 degrees, under the supervision of the examiner who monitors this angle. Throughout the jump, the trunk is maintained in a vertical position to the best extent possible, and the athlete is instructed to generate maximum height and perform the jump swiftly. Participants performed the jump with their arms crossed over the rib cage and hands resting on their shoulders to minimize the potential influence from the upper limbs (25).
3.5. Kinetics Evaluation
The data were captured using a force platform (Kistler, Switzerland, type: 523342) that recorded the vertical ground reaction force data at a sampling frequency of 1200 Hz. From the conducted trials, only the jump with the greatest height was chosen for subsequent analysis. Jump height was determined using the following equation: h = t2 × 1.22625, where h represents the jump height in meters and t denotes the flight time of the jump in seconds (28).
MF was defined as the peak value attained during the positive acceleration phase of the CMJ (25). The positive acceleration phase commenced when the vertical force trace exceeded the participant's body mass subsequent to the countermovement phase of the jump. Rate of force development during the jump was determined as the force-time record difference between the point of maximum vertical force and the initiation of the positive acceleration phase, divided by time, and expressed in N·sec-1 (29). Time to maximum force (TMF) referred to the duration it took for the positive acceleration phase to reach the point of maximum force (30).
3.6. Statistical Analysis
All statistical analyses were performed using SPSS version 25. The data are presented as means ± SD. The normality assumption was assessed through the Shapiro-Wilk test, complemented by a visual examination of histograms and QQ plots, as well as calculations of skewness and kurtosis. Additionally, Mauchly's test was utilized to examine sphericity.
A two-way repeated measures ANOVA was performed with MF and RFD as the dependent variables. The within-subjects variable was 'timepoints' (before the intervention, after the intervention, and follow-up), and the between-subjects variable was 'condition' (tDCS, sham, and control). The main effects of 'time' and 'condition,' as well as the interaction effects of time × condition, were analyzed for MF and RFD. Since the normal distribution assumption was violated, the Friedman test was employed to assess the impact of conditions on TMF; for Friedman's test, effect size estimation was based on Kendall's W coefficient of concordance (31). Partial eta squared
4. Results
To assess the test-retest reliability of the force plate measurements for the variables under investigation, participants conducted two maximum CMJs on the force plate during their initial visit to the laboratory. The intra-class correlation coefficient (ICC) was computed to evaluate test-retest reliability. The findings indicated excellent reliability for the variables of MF (ICC = 0.98), TMF (ICC = 0.98), and RFD (ICC = 0.99).
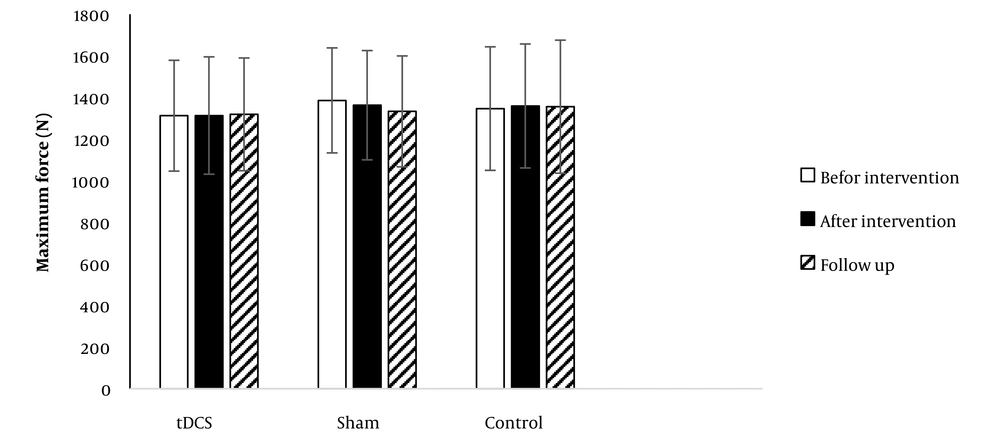
4.1. Maximum Force
Figure 2 shows data obtained for MF in CMJ in all groups. No significant effect was detected for time (F = 1.324; P = 0.271;
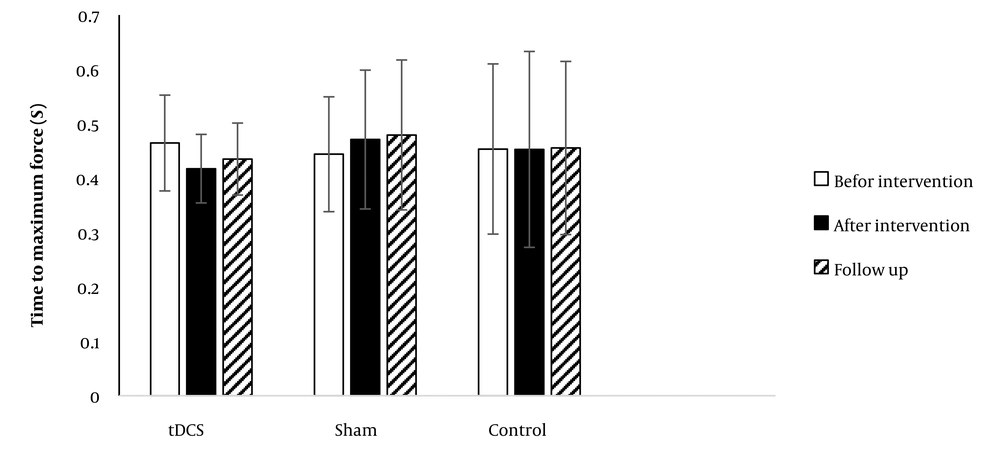
4.2. Time to Maximum Force
Figure 3 presents the data obtained for TMF in the CMJ across all groups. Given the violation of the normality assumption, the Friedman test was utilized to assess differences. The test did not demonstrate any significant differences in TMF among the different conditions (
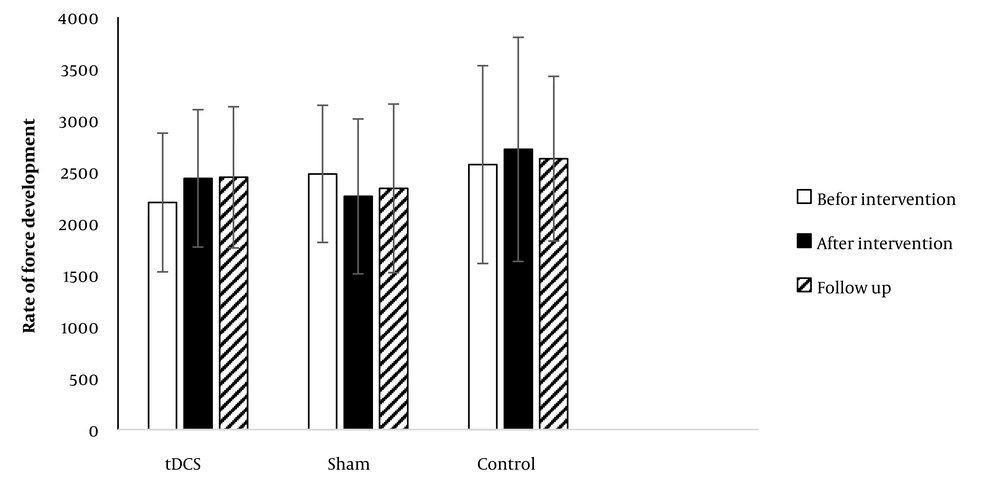
4.3. Rate of Force Development
Figure 4 shows data obtained for RFD in the CMJ in all groups. No significant effect was detected for time (F = 0.334; P = 0.717;
These results indicate that tDCS does not affect MF, TMF, and RFD in the CMJ of non-elite jumping athletes.
5. Discussion
The primary objective of this study was to examine the impact of applying 20 minutes of a-tDCS at 2 mA over the Cz region on MF, TMF, and RFD during the CMJ task in non-elite jumping athletes compared to the Sham and control conditions. Our findings indicated no statistically significant differences in these variables among the tDCS, Sham, and control groups.
This finding aligns with the findings of Romero-Arenas et al. (32), who demonstrated that the application of 15 minutes of a-tDCS at 2 mA over the dorsolateral prefrontal cortex did not lead to improvements in the CMJ performance. In their study, 17 healthy men underwent three experimental conditions (a-tDCS, c-tDCS, and sham) at one-week intervals. Before and after each intervention session, participants performed three CMJs. They found no significant differences between the conditions for CMJ height and muscular peak power. Our results are consistent with numerous studies (33-35) that have also reported non-significant improvements in sports performance following tDCS use. In a meta-analysis conducted by Holgado et al. (36), which considered mixed and conflicting reports, it was concluded that if tDCS does have any effect on exercise performance, it is likely to be small and influenced by publication biases.
In contrast to our findings, Lattari et al. (18) observed improvements in the CMJ performance following the application of 20 minutes of a-tDCS at 2 mA over the Cz region. In their study, ten athletes participated in three testing conditions (a-tDCS, c-tDCS, and sham), with a one-week interval between each session. Prior to and following each intervention session, participants performed three CMJs. The authors reported significant increases in jump height and peak power after a-tDCS. However, it is worth noting that Button et al. (37) have demonstrated that statistically significant effects observed in studies with a small number of participants may easily be indicative of false positives. Given that the current study had a larger sample size (48 participants) compared to Lattari et al.'s (20) study (10 participants), this discrepancy in results may be attributed to the difference in sample sizes.
Some studies have also reported improvements in physical performance following the application of a-tDCS (21, 22, 38). In our view, several reasons could account for these conflicting results. Variations in electrode montages and stimulation protocols could contribute to different findings (39). Additionally, due to differences in electrode size, positioning, and the relatively limited focal specificity of the induced electric field, tDCS may affect areas of the brain beyond the intended target, which can significantly impact the outcomes (40). The type of muscle contraction or the size of the recruited muscle mass could also influence the requirements of the motor cortex, thereby affecting the amplitude of response (23). Another factor, as explained by Sidhu et al. (41), is that the effects of multi-joint exercises on the response of corticospinal cells differ significantly from those of single-joint exercises. The responsiveness of neurons within the human brain is likely dependent on the specific physiological responses elicited by different exercise modes.
Interestingly, in recent years, there has been a notable decline in the observed effects of tDCS on cortical excitability. This decline could be attributed to technological and methodological advancements since 2000, which have likely minimized noise levels and increased the reliability of more recent outcome measures (42). However, it is worth noting that some studies have shown that even if cortical excitability increases following a-tDCS, this increase does not necessarily translate into improvements in sports performance (35). Additionally, Montenegro et al. (23) suggested that the influence of a-tDCS may be more pronounced in patients with motor cortex hypo-excitability disorders rather than in healthy individuals. This can be explained by the theory that in healthy individuals, there may be a ceiling effect of tDCS on cortical excitability (43).
Furthermore, the reliability and reproducibility of traditional tDCS have recently come under scrutiny. Wiethoff et al. (44) conducted a study that demonstrated the variability in response to tDCS, particularly the polarity-dependent effect. They found that among the participants in their study, 50% either responded poorly or did not respond at all to tDCS. Furthermore, among those who did respond, 21% exhibited an "inverted typical response," wherein a-tDCS led to suppression of corticospinal excitability while c-tDCS increased it. The authors attributed this variability in response to tDCS to various individual differences, including factors such as anatomy, neurochemistry, neurophysiology, psychological state, gender, and genetics.
5.1. Conclusions
The findings of our study indicate that the application of 20 minutes of a-tDCS at 2 mA over the Cz region did not have a significant impact on MF, TMF, and RFD in the CMJ task among non-elite jumping athletes when compared to the Sham and control conditions. These results suggest that the tDCS protocol used in this study, including the intensity and electrode placement, may not be sufficient for improving the kinetic variables of the CMJ in non-elite jumping athletes. However, it is important to note that these findings do not dismiss the potential use of a-tDCS in modified strength training programs for individuals who are unable to engage in high-intensity training.
5.2. Limitations
This study has some limitations. While electrical stimulation was targeted to specific areas of the lower limbs, it is possible that other areas of the cortex were influenced due to the electrode size. In other words, determining the precise location of electrical stimulation was limited. Additionally, this study did not examine the neurophysiological effects of tDCS on brain cell activity, which is another limitation to consider.
.png)