1. Background
Osteoarthritis (OA) of the tibiofemoral joint is a common cause of pain and disability in the elderly (1). The prevalence of OA on the tibiofemoral joint increases with age. As many as 30% of the individuals older than 45, and nearly 75% of the individuals older than 65 show an evidence of OA of the tibiofemoral joint on knee radiographs (2, 3). Furthermore, 40% - 80% of the individuals with radiographic evidence of OA have a symptomatic disease (4).
OA produces great impact on pain, loss of mobility and progressive amelioration on the quality of life (QoL) (5, 6). It is the cause of 50% of the disabilities in Spain (7). Besides, the direct cost of OA in Spain represents 4,738 millions of euros per year (7).
The normal knee is composed of cartilage, subcondral bone, synovial tissue and articular capsule (8). In OA of the knee, there is destruction of the articular cartilage, narrowing of the articular space, sclerosis of the subchondral bone, osteophyte formation and subchondral cysts (1, 8).
Radiography is usually the initial imaging examination performed in patients with OA of the tibiofemoral joint. Radiography is also commonly used in population studies to define the presence of OA of the femoral joint and to document the changes in the severity of the disease process over time (1). The articular cartilage gets thin and swollen as OA progresses, but normally, cartilage thickness diminishes with time on knee OA (9). The progression is greater in the medial compartment compared to the lateral compartment of the tibiofemoral joint. Based on this assumption, there is a moderate correlation between joint space narrowing (JSN) and the loss of articular cartilage (10-12). JSN is defined when the minimal joint space width is less than 3 mm for the tibiofemoral joint (13). Since cartilage is not seen on radiography, its loss is indirectly measured by the narrowing of the articular space (14). Therefore, radiography is a costless method to monitor the OA progression, and it is currently the gold standard, that is, the accepted and the simplest method to evaluate the OA progression and cartilage destruction (15-20).
There is no cure for OA nowadays (8). The main goals in OA are to ameliorate the symptoms of the disease such as pain, rigidity, inflammation, and ideally, to diminish or prevent articular damage and joint destruction (8, 21-23).
Knee OA is considered a multifactorial, active disease driven by both biomechanical and proinflammatory factors (8, 24, 25). It is believed that inflammation plays an important role in Knee OA in such an extent that future treatments should act on the regulation of inflammation to diminish the progression of OA (8).
Recently, Fernandez-Cuadros et al. have postulated Ozone as a feasible future treatment on knee OA based on decades of clinical experience and several in vivo and in vitro studies on the modulation of inflammation (8). Moreover, Ozone could act on several therapeutic targets involved in the pathophysiology of Knee OA (such as mineral metal proteases, nitric oxide, prostaglandin E2, pro-inflammatory and anti-inflammatory cytokines, growth factors and stem cells), besides inflammation (8). Several authors worldwide state that pain, function and quality of life improve significantly after the Ozone therapy. However, to the best of our knowledge, there is no study on the effect of Ozone on joint space width, which is an important feature of the progression of the disease.
2. Objectives
The objective of our study is to evaluate the effect of Ozone on pain, function, quality of life, minimal joint space and knee arthroplasty delay in a case series of patients with knee Osteoarthritis (OA).
3. Methods
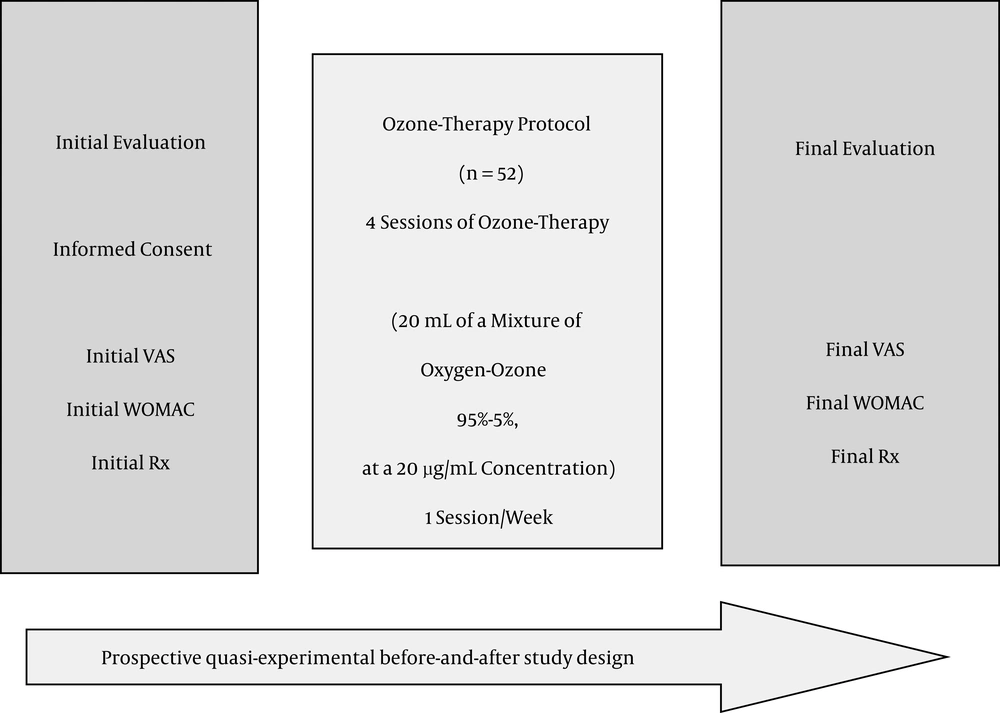
A prospective before-and-after quasi-experimental study was performed on 52 patients out of 120, with knee OA Kellgren-Lawrence (K-L) grade 2 or more (Figure 1). The follow-up period has been two years (from January 2014 to June 2016) to patients who attended the rehabilitation department at Santa Cristina’s university hospital. All patients were given previous medical and rehabilitation treatment but without symptomatic improvement. The study was approved by the hospital ethical committee.
Note: VAS, Visual Analogical Scale; WOMAC, Western Ontario Mc Master Universities Index for Osteoarthritis; Rx, posterior anterior weight bearing plain radiography with the knees fully extended; protocol applied at the department of rehabilitation at Santa Cristina’s university hospital; the study ran from January 2014 to June 2016.
Inclusion criteria: 1) patients with knee OA, K-L grade 2 or more; 2) with pain greater than 3 on the VAS scale; 3) who have failed all other conservative treatments (Non anti-inflammatory steroidal drugs, rehabilitation, physical therapy); 4) and are unwilling to or not candidates for knee arthroplasty replacement; 5) and are older than 18 years of age.
Exclusion criteria: 1) patients with any formal contraindication to Ozone therapy (favism, pregnancy, angiotensin converting enzyme inhibitors treatment, hyperthyroidism, thrombocytopenia, serious cardiovascular instability and allergy to Ozone); 2) patients who failed to complete the whole Ozone therapy treatment protocol; 3) patients who failed to fill any of the questionnaires applied (VAS/WOMAC 4) cases with absence of weight-bearing plain radiographies before-after the treatment.
On the initial evaluation, age, comorbidities, occupation and other demographic data were obtained. An explanation of the ozone treatment protocol was given and Informed consent was obtained. Initial WOMAC index and initial weight bearing bilateral radiographies were taken. The ozone protocol consisted of four sessions (1 session/week) of an intra-articular infiltration of a medical mixture of oxygen-ozone (95% - 5%) at a 20 µg/mL concentration. A 27G, 4 cm Quincke needle was used to deliver Ozone into the joint. The skin was previously cleaned with 1% Chlorhexidine. With the knee semi-flexed, Ozone was slowly infiltrated on the lateral aspect of the knee next to the patella, with mild patella subluxation to expose the articular joint space. After the infiltration, the knee was flexed and extended several times in an attempt to distribute the oxygen-ozone mixture all over the articulation and the lateral recesses, and to confirm that the infiltration was delivered into the articulation by listening to a crepitus noise (Perez-Moro Maneuver).
After four sessions were performed, a control with WOMAC index and weight-bearing bilateral radiographies were taken, one-two months after the treatment. From that point on, evaluations were accomplished every 6 months, depending on the clinical symptoms. If treatment was necessary, a new 4-session protocol was applied and control radiographies were performed afterwards.
The symptoms severity of OA was evaluated using the WOMAC Index (3, 26, 27). The radiographic severity of knee OA was assessed using the K-L grading system, which relied on specific radiographic findings: presence of osteophytes, joint space narrowing, and subchondral sclerosis (13, 28, 29).
The WOMAC Index contained 24 questions in a total of three sections, namely pain, stiffness and function (13, 26, 27). Each section had five response options (none, mild, moderate, severe and extreme), and subtotal scores for pain (five Items), stiffness (two Items) and function (17 Items) ranged from 0 - 20, 0 - 8 and 0 - 68, respectively.
The K-L grades were defined as follows: Grade 0, no features of OA; Grade 1, small osteophyte of doubtful importance; Grade 2, definite osteophyte but an unimpaired joint space; Grade 3, definite osteophyte with moderate diminution of joint space; and Grade 4, definite osteophyte with substantial joint space reduction and sclerosis of subchondral bone (13, 28, 29).
For the radiographic evaluation of the medial and lateral tibiofemoral joint, bilateral posterior-anterior radiographies with both legs standing and fully extended in a weight-bearing condition, were executed. All radiographic images were digitally acquired using a picture archiving communication system (PACS). To avoid errors, radiographs on the medial and lateral tibiofemoral compartments, on the same minimal joint space width interval, for both compartments were evaluated by one of the authors before and after the treatment and on the same day of the follow-up evaluation. That is, we measured the inter bone distance on the radiograph in the medial and the lateral compartments, marked each by a pair of points at the perceived narrowest distance of the joint space, using the PACS measuring program. All assessments were carried out by just one author, in order to reduce the inter-observer variation whose coefficient of variation for repeated measures is 3% - 8% (30).
We considered a difference greater than 6% of the maximum scores of the WOMAC Index as being clinically important. That is, for the WOMAC: pain = 1.2; stiffness = 0.5; function = 4.1. Statistical analyses were conducted using the SPSS® version 20.0. To initially evaluate the quantitative and qualitative variables; frequencies, means and percentages were used. To evaluate a significant change before-and-after the treatment, t-student test for quantitative variables was used; while for the evaluation of the qualitative variables, x2 test was used. The level of significance was 99% (P = 0.01). If a new treatment cycle was needed, repeated measurement for statistical analysis was applied.
4. Results
In this study, 52 out of 120 patients have been studied. Ten patients were male (19.2%), while 42 (80.8%) were female, with a male to female ratio of 1:4 (Table 1).
| Variable | Analyzed Value |
|---|---|
| Female, n (%) | 42 (80.8) |
| Male, n (%) | 10 (19.2) |
| Ratio female:male | 4:1 |
| Age, years ± SD | 70.36 ± 10.3 |
| Follow-up period, months | 10.2 |
| Kellgren-Lawrence 2° grade, n (%) | 6 (11.5) |
| Kellgren-Lawrence 3° grade, n (%) | 36 (69.2) |
| Kellgren-Lawrence 4° grade, n (%) | 10 (19.3) |
| Visual analogical scale (0 - 10) n ± SD | 8.19 ± 1.2 |
| WOMAC pain-subscale (0 - 20) n ± SD | 16.5 ± 2.2 |
| WOMAC stiffness-subscale (0 - 8) n ± SD | 3.2 ± 2.7 |
| WOMAC function-subscale (0 - 68) n ± SD | 48.1 ± 13.6 |
Abbreviations: WOMAC, western ontario and mc master universities index for osteoarthritis; SD, standard deviation.
The mean age of the population was 70.36 years, with people ranging from 45 to 93 years of age (Table 1).
The most frequent K-L grade of the patients analyzed was 3° grade (n = 36, 69.2%), (Table 1).
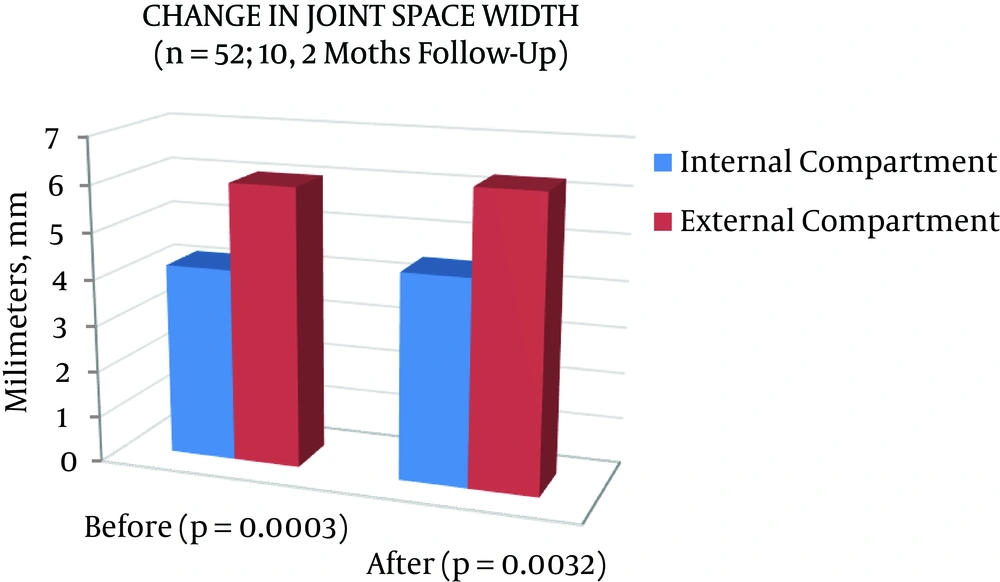
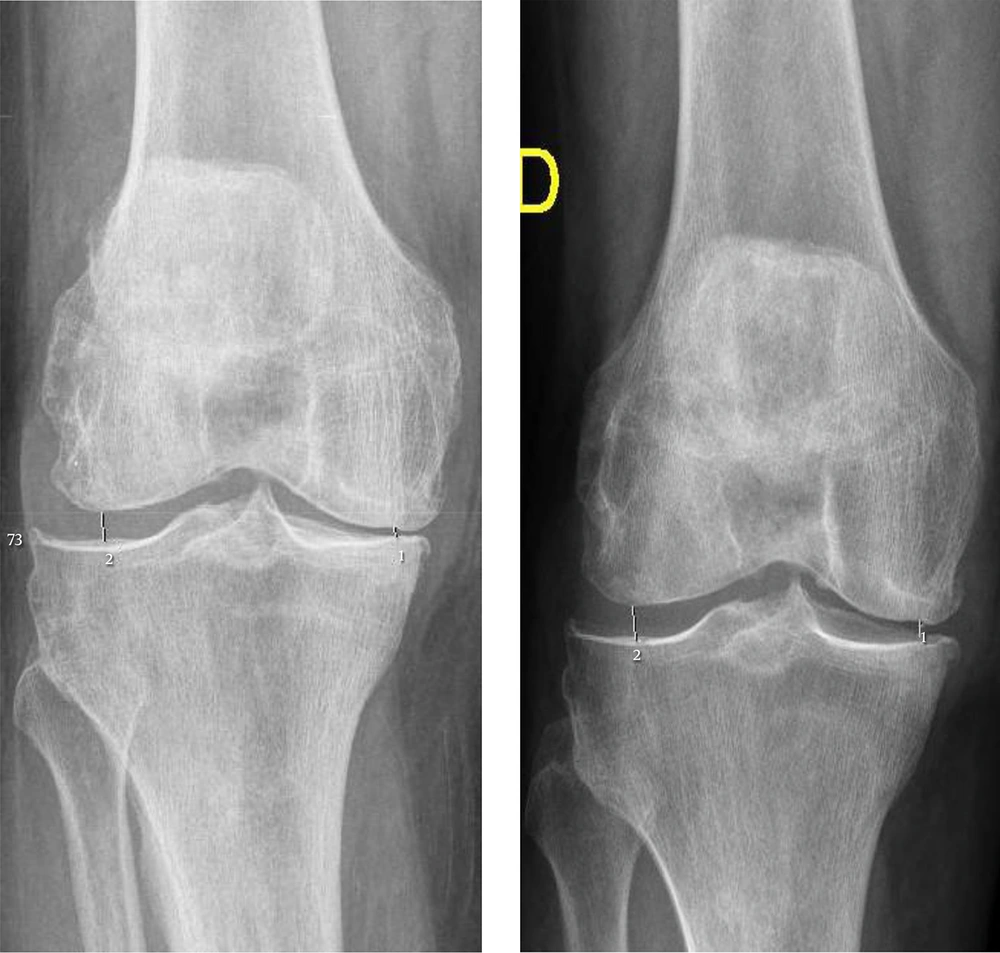
On the evaluation of the minimal joint space in the different tibiofemoral compartments in this population, the internal compartment measured 4.17 mm and increased significantly to 4.44 mm (P = 0.0003); while the external compartment was 6.02 mm and improved significantly to 6.26 mm (P = 0.0032) after the protocol treatment (Table 2, Figures 2 and 3).
| Knee OA | Medial Tibiofemoral Compartment | Lateral Tibiofemoral Compartment | ||||
|---|---|---|---|---|---|---|
| Before Mean (± SD) | After Mean (± SD) | P | Before Mean (± SD) | After Mean (± SD) | P | |
| K-L 2° (n = 6), mm | 5.25 ± 1.17 | 5.11 ± 1.61 | 0.6727 | 5.13 ± 0.78 | 5.36 ± 0.71 | 0.2403 |
| K-L 3° (n = 36), mm | 4.25 ± 1.13 | 4.57 ± 1.10 | 0.0002 | 6.06 ± 0.92 | 6.28 ± 1.04 | 0.0170 |
| K-L 4° (n = 10), mm | 3.21 ± 1.86 | 3.55 ± 1.73 | 0.0235 | 6.36 ± 2.28 | 6.75 ± 2.27 | 0.1815 |
| Global (n = 52), mm | 4.17 ± 1.39 | 4.44 ± 1.35 | 0.0003 | 6.02 ± 1.29 | 6.26 ± 1.36 | 0.0032 |
Abbreviations: OA, Osteoarthritis; K-L, Kellgren-Lawrence Scale for Osteoarthritis; SD, Standard Deviation.
If we consider the K-L grades and the tibiofemoral compartments, there are some subtle differences. On the internal compartments, the greater the K-L degrees, the smaller the joint space width. However, for the external compartment, the greater the K-L degree, the greater the joint space width. After the treatment, both internal and external compartments significantly increase their minimal joint space width. If K-L degrees are considered, all compartments (internal and external) increased joint space width significantly, except for K-L 2° degree, which decreased joint space width, but not significantly (Table 2).
Regarding the pain, function and stiffness evaluated by VAS and WOMAC scales, all patients significantly improve both scales after the treatment. If the different K-L degrees are considered, Stiffness Subscale increased notably in K-L 4° degree before the treatment. Nevertheless, all patients significantly improved pain, function and stiffness after the Ozone treatment (Tables 3 and 4).
| Knee OA | VAS (0-10) | WOMAC Pain (0-20) | ||||
|---|---|---|---|---|---|---|
| Before Mean (± SD) | After Mean (± SD) | P | Before Mean (± SD) | After Mean (± SD) | P | |
| K-L 2° | 8.6 ± 1.2 | 5.1 ± 3.3 | 0.0127 | 17.1 ± 2.5 | 9.6 ± 6.9 | 0.0092 |
| K-L 3° | 8.0 ± 1.2 | 2.1 ± 1.8 | 0.0000 | 16.3 ± 2.0 | 4.1 ± 3.0 | 0.0000 |
| K-L 4° | 8.5 ± 1.1 | 2.3 ± 2.2 | 0.0000 | 16.8 ± 2.6 | 4.8 ± 4.3 | 0.0000 |
| Global | 8.1 ± 1.2 | 2.5 ± 2.3 | 0.0000 | 16.5 ± 2.2 | 4.9 ± 4.1 | 0.0000 |
Abbreviations: OA, osteoarthritis; VAS, visual analogical scale; WOMAC, western ontario and Mc master universities index for osteoarthritis; K-L, Kellgren-Lawrence scale for osteoarthritis.
| Knee OA | WOMAC Stiffness (0-8) | WOMAC Function (0 - 68) | ||||
|---|---|---|---|---|---|---|
| Before Mean (± SD) | After Mean (± SD) | P | Before Mean (± SD) | After Mean (± SD) | P | |
| K-L 2° | 2.8 ± 3.4 | 1 ± 1.1 | 0.1204 | 39.5 ± 23.9 | 15.8 ± 8.5 | 0.0260 |
| K-L 3° | 2.7 ± 2.6 | 0.8 ± 1.5 | 0.0000 | 49.3 ± 11.7 | 17.2 ± 16.5 | 0.0000 |
| K-L 4° | 5.2 ± 1.8 | 2.1 ± 1.6 | 0.0005 | 48.6 ± 11.8 | 20.0 ± 14.1 | 0.0001 |
| Global | 3.2 ± 2.7 | 1.0 ± 1.5 | 0.0000 | 48.0 ± 13.6 | 17.6 ± 15.2 | 0.0000 |
Abbreviations: OA, osteoarthritis; WOMAC, western Ontario and Mc master universities index for osteoarthritis; K-L, Kellgren-Lawrence scale for osteoarthritis.
5. Discussion
OA is one of the most disabling and incapacitating diseases on the autonomy of older people. OA produces great impact on pain, function and the use of resources (6, 8). OA is considered a problem of public health. Nowadays, there is no cure for it. For that reason, the goals of the treatment in the short term are to ameliorate symptoms (by symptomatic slow acting drugs for OA or SYSADOA) and in the long term to diminish or to revert articular damage and joint destruction (by disease modifying drugs for OA or DMDOA) (7, 8). Several authors state that Ozone is effective in ameliorating the pain and improving the function and the quality of life. However, to the best of our knowledge, there is no report of Ozone as a DMDOA, despite the multiple studies (8) that date clinical benefit in knee OA.
OA increases exponentially with age. The average age of 70 years in our study is in accordance with those of Fernandez-Cuadros (6), O’Brien (31), Bachmeier (32) and Ramon-Rona (33). In our study, OA was more frequent in women, with a male to female ratio of 1:4. This coincides with published articles by Ramon-Rona (33) and Moreno-Palacios (34). In fact, females are at a higher risk of presenting hip, knee and hand OA. Some studies report lower joint space width (JSW) and higher narrowing in females (35, 36).
This is the first study that states the chondroprotector effect of Ozone on knee OA, evaluated by radiographs, on an average follow-up period of 10 months to a maximum of 28 moths in some cases. Because of the symptoms amelioration, none of the patients underwent total knee replacement treatment. This evidence is important, because to demonstrate the DMDOA effect of Ozone (that is, the slowing or the reversal of the progression of OA), it is necessary to perform prospective studies in long periods of time, ideally one-two years or more, with radiographic follow-up to date such an improvement or slowing in joint degeneration (7).
To date, researchers have failed to develop effective and safe DMDOA, because the pathogenesis of OA is not fully understood (37, 38).
In our study, after a maximum of a 28 month follow-up, none of the patients performed a knee replacement arthroplasty. In knee OA, both patients and doctors sustain that postponing the surgery is a success in OA treatment (37, 38). In that scenario, Ozone was capable of that achievement. Joint replacement is the final treatment option for knee OA (37); and, although total knee arthroplasty is the orthoprostetic operation with the highest clinical success rate, good prognosis and sustained results after 10 years in 95% of the patients (6), this intervention is an expensive and invasive surgical procedure which is not exempt of side effects and complications (5, 8).
Life-time risk for knee OA is 45% and life-time risk for total knee replacement is 6% - 7%, based on the results from the UK general practice research database. Even so, 32% of the patients considered for total knee arthroplasty replacement were unwilling to consider surgery as an option (39). In such cases, Ozone is a treatment option.
Radiological JSW is the current “gold standard” and has been recommended as the best available method for assessing the anatomical progression of OA in the studies of arthritis. A change in the tibiofemoral JSW is recommended as the primary measure of biological change in OA, and indirectly, the primary measure to evaluate the biological treatments in OA (19, 20, 40).
Sensitive and accurate methods for measuring the joint damage in OA are essential for the assessment of disease progression and for the development of DMDOA (18, 41). Although MRI is considered the method of choice to accurately monitor the cartilage changes in OA, the measurement of JSW from radiographs is currently the simplest and the most costless way to evaluate the progression of cartilage destruction in OA (16-18). However, JSW varies with weight-bearing, alignment of the medial tibial plateu, x-ray beam inclination, rotation of feet and the degree of knee flexion (15, 18, 42, 43). That is the reason to use plain radiographies in weight-bearing position with knees fully extended in order to avoid such confounding factors and errors in the measurement of the compartments in our study.
As a resume, it can be stated that JSN is a surrogate marker of articular cartilage volume loss and an indicator of structural change in OA (19, 40). JSN can be used as an indicator of OA progression. In fact, there is a linear negative correlation between cartilage volume loss and JSN grades. It is estimated that JSN grade 2° still benefits from chondroprotective measures, because this grade still has significant amounts of articular cartilage (40).
Boegard in a 2-year follow-up observational study has noticed that the mean minimal JSW diminishes in the medial tibiofemoral compartment, while the same space increases in the lateral tibiofemoral compartment. This was observed in a sample of 55 patients with and without OA with an age range from 35 to 54. The average difference of space narrowing was 0.2 mm in a 2-year follow-up study (from 3.01 to 2.81 mm) (43).
Ledinghan et al. and similarly Boegard in a 2-year follow-up observational study, in people with knee OA observed that an increase in the JSW was only seen in the lateral tibiofemoral compartment and corresponded with narrowing of the contralateral (medial) worst affected compartment (44).
Lanyon et al. have reported the narrowing of both medial and lateral tibiofemoral compartments, in a 3-year follow-up study, in 51 people aged on average 71 (43). Boegard stated that in a 2-year follow-up study, the annual ratio of the narrowing of minimal JSW was 0.13 mm (43). Cicuttini, in an observational study, in 28 subjects of an average age of 62, observed an annual rate of JSN of 0.24 ± 0.29 mm (from 7.81 ± 4.1 to 7.3 ± 4.5 mm) (20). Buckland has observed a rate of change in semi flexed radiographs of the JSW of 0.26 mm/year (30).
All previous authors state that normally patients with knee OA lose 5% of tibiofemoral cartilage per year (20). When the first radiological changes on OA are detected, 13% of knee cartilage has already been lost (45). That loss of cartilage correlates with the worsening of symptoms and predicts knee replacement (46).
Some studies have demonstrated the DMDOA effect of chondroitin sulfate as a chondroprotector drug, slowing the annual rate of cartilage loss to 0.04 - 0.05 mm/year compared to the normal cartilage loss of 0.32 to 0.4 mm/year in control groups (7, 47, 48). There are only two studies that demonstrate an increase of the minimal joint space to 0.02 - 0.1 mm/year compared to the normal cartilage loss rate of 0.04 - 0.4 mm/year (49, 50).
To the best of our knowledge, this is the first study that states the chondroprotector or DMDOA effect of Ozone, increasing significantly both the medial and the lateral tibiofemoral compartments from 4.17 mm to 4.44 mm) and mm (+ 0.27 mm) and from 6.02 mm to 6.26 mm (+ 0.24 mm) respectively in an average 10-month follow-up period (ranging from 3 to 28 months). This positive chondroprotector effect has been observed in all K-L degrees and in both medial and lateral tibiofemoral compartments except for the medial compartment in K-L 2° grade, but the difference is not significant (P = 0.6727). Probably, the non-significant effect on K-L 2° grade is because of the small sample size in that specific group (n = 6).
All K-L grades have improved on pain and function after Ozone treatment, measured by VAS and WOMAC scales. There is a positive correlation between pain and function improvement and radiological minimal JSW improvement on both medial and lateral tibiofemoral compartments.
In our study there is a negative correlation between K-L grade and JSW. That is, in lower K-L grades, there is a greater JSW and while OA progresses, the JSW diminishes. That correlation is only valid for the medial tibiofemoral compartment. On the other hand, there is a positive correlation between K-L grade and the JSW. That is, as OA progresses, the lateral compartment increases ought to the narrowing of the medial compartment which is normally the most affected compartment, a phenomena that was clearly stated by Ledingham (44). Despite this consideration, both medial and lateral compartments increase their JSW after the Ozone treatment; that structural positive change in an OA joint is an indirect demonstration that Ozone has a chondroprotector and reparative effect on articular cartilage and subchondral bone, and that Ozone acts on both compartments of the damage joint.
Pain, loss of function and loss of articular cartilage are predictive factors for the replacement of total knee joint. Ozone has positive effect on symptoms such as pain and function (as recently stated by Fernández-Cuadros et al) (51), and it has chondroprotector effect on articular cartilage and subchondral bone, increasing the minimal JSW, as it is observed in our prospective study. The sum of both effects let doctors delay total knee replacement in patients treated by Ozone at least in our average 10-month follow-up study (ranging from 3 to up to 28 months).
From the clinical point of view, the great impact of this study is that Ozone preserves and probably restitutes articular cartilage, delaying the need for total knee arthroplasty replacement.
5.1. Study Limitations
An important Limitation of the study is the lack of control group. This is mainly due to the limited number of cases (n = 52). As the effectiveness of Ozone in the control of pain in knee OA has been demonstrated for decades and all patients accepted the proposed treatment protocol, it is not ethical to deny Ozone intervention. A quasi-experimental before-after study (also referred to as a non-randomized control trial) is applied in this specific ethical situation in order to solve the lack of control group and to give clinical-based evidence. In such a case, a pretest-post-test is performed on the same treatment group, and the change observed after the intervention is expected as a direct consequence of the Ozone treatment protocol.
5.2. Conclusion
Ozone treatment is capable of producing pain relief, function recovery and radiological improvement on minimal joint space in knee OA patients.
From the results of our study, it is assumed that Ozone could slow/revert OA progression, due to the increase in the minimal internal and external joint space width.
Ozone treatment delays the need for total knee arthroplasty.