1. Background
During the past 3 decades, mankind has been exposed to many new physical and chemical agents. A major physical agent is electromagnetic field (EMF), which among other agents, is widespread and ubiquitous in modern daily life. Therefore, most people are exposed to ELF-EMF (extremely low frequency electric and magnetic fields) emitted by power lines, electrical panels, transformers, and domestic, electrical, and electronic devices (1-3). Associations between exposure to ELF-EMF and possible health hazards to mankind have been investigated in several epidemiological and experimental studies (4-8). Most of the epidemiological studies have shown that exposure to ELF-EMF has increased the incidence of certain types of cancer, especially acute childhood leukemia (9-11). Furthermore, several in vitro and in vivo studies have been published on the biological effects of ELF-EMF and some hypothetical mechanisms have been detected (12). A limited number of these studies have demonstrated that ELF-EMF promotes carcinogenic effects. Some available evidence appears that exposure to ELF-EMF can enhance or inhibit cell proliferation and apoptosis (13, 14). Several studies indicated that ELF-EMF exposure affects cell regulation and chromosomal structure (15-17). Moreover, other in vitro studies suggested that ELF-EMF can change the expression of some protein, which is involved in the control of cell proliferation (18, 19), while others demonstrate that exposure to ELF-EMF has no effects on cell proliferation, DNA replication, and cell regulation (20). As it seems, there exist some ambiguities in results. Some of these conflicting data might be due to the difference in frequency, intensity, duration, and also a certain type of cell lines (21).
A long-term study on the effects of ELF-EMF on the fertility and tumor promotion process has shown that exposure to 50 Hz magnetic fields is a significant risk factor for neoplastic development and infertility in mice; however, it has not increased the incidence of liver and lung tumor (22). It has shown that exposing liver cancer BEL-7402 cells to folic acid-modified magnetic nanoparticles (FA-MNPs) in the presence of 100 Hz ELF-EMF, significantly inhibited cell proliferation and induced higher apoptosis (23). Besides, it has shown that ELF-EMF exposure (50 Hz, 8 mT, 28 days) causes significant deterioration in learning and memory abilities (24).
The aim of this study was to determine the effects of ELF-EMF (50 Hz) on the rats’ liver tissue exposed to different intensities (0.5 and 1 mT) at different time durations (2 and 4 weeks) by analyzing microscopic changes in liver tissue.
2. Methods
2.1. Animals
Adult male Wistar rats, weighing 250 - 300 g were housed 3 to 4 per cage in a temperature-controlled colony room under light/dark cycle and had free access to water and food throughout the experiment. It is notable that the rats were exposed to these intensities 8 h/day and were housed in normal lighting conditions (12 hour light ON, 12 hour light OFF). This study was conducted in accordance with the policies stipulated in the guide for the care and use of laboratory animals (NIH) and was approved by the local ethics committee of Semnan University of Medical Sciences.
2.2. Exposure System
A calibrated ELF/EMF generator was used to produce an electromagnetic field. It was able to generate homogenous sinusoidal ELF-EMF, with the intensity of 0.5 and 1 mT as well as a frequency of 50 Hz with a 40 cm diameter Helmholtz coil placed around the exposure cage (Figure 1) 8 hrs/day for 2 and 4 weeks. Control rats were placed in the cage outside the coil. Sham exposed rats were maintained for an equal period of time inside the exposure cage with the generator off. The room was maintained at a constant temperature (25 ± 1.0°C) for all experiments.
2.3. Experimental Groups
The experimental groups consists of the following, in which 5 rats were considered in each group: Eexposure to 0.5 mT for 2 week (A), exposure to 0.5 mT for 4 week (B), control without radiation (which was not placed at the experimental condition) (C), exposure to 1 mT for 2 week (D), exposure to 1 mT for 4 week (E), and sham group (which was placed at the experimental setup cage with no exposure to consider the probable stress induced effects on the results) (F).
2.4. Slide Preparation
For the Histopathological assessment, the liver tissue was removed from anesthetized rat, fixed in 10% formaldehyde and embedded in paraffin wax. Then, 3-5 µm sections of tissue were cut and stained with Hematoxylin and Eosin (H and E). The prepared slides were used for light microscopy analysis in the 10 × and 40 × fields. In order to classify different experimental groups concerning cellular damage and tissue pathology, several parameters such as focal or distributed apoptosis, focal or distributed necrosis, portal, periportal or parenchymal inflammation (focal or distributed), and hepatocitolysis were assessed in different experimental groups by an anatomical pathologist who was blind to the groups.
3. Results
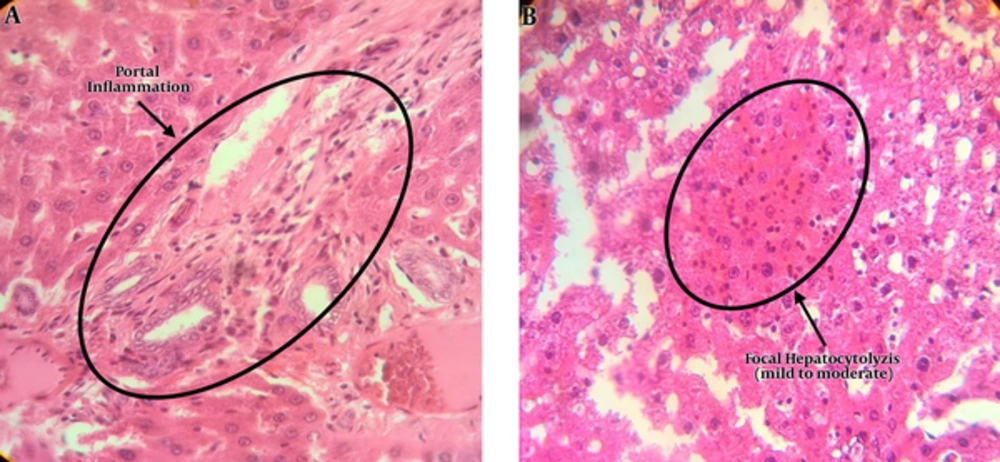
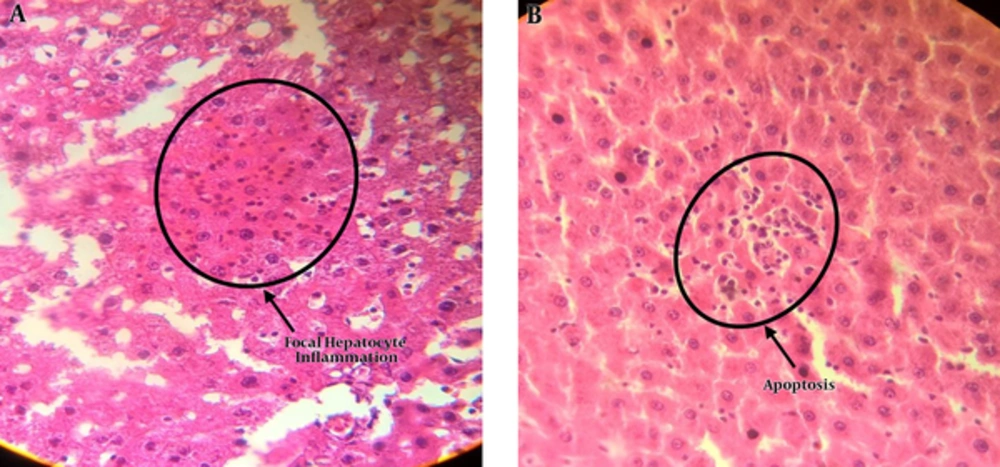
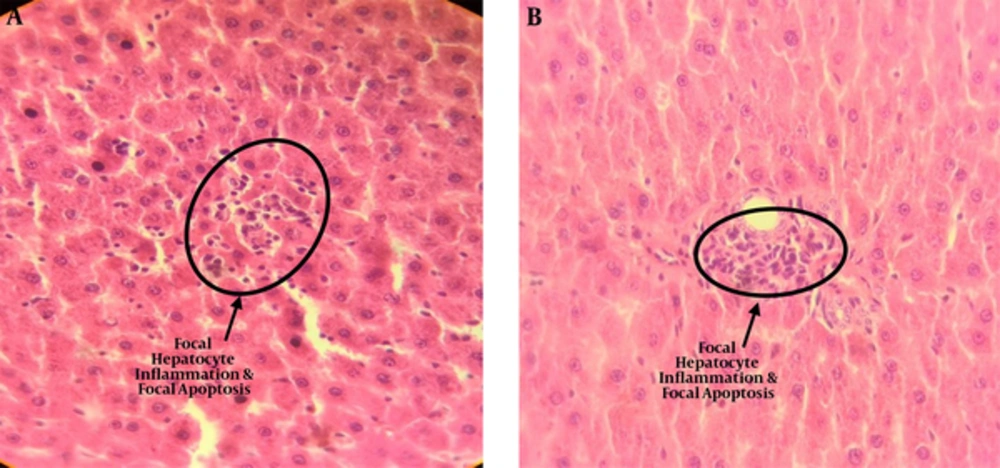
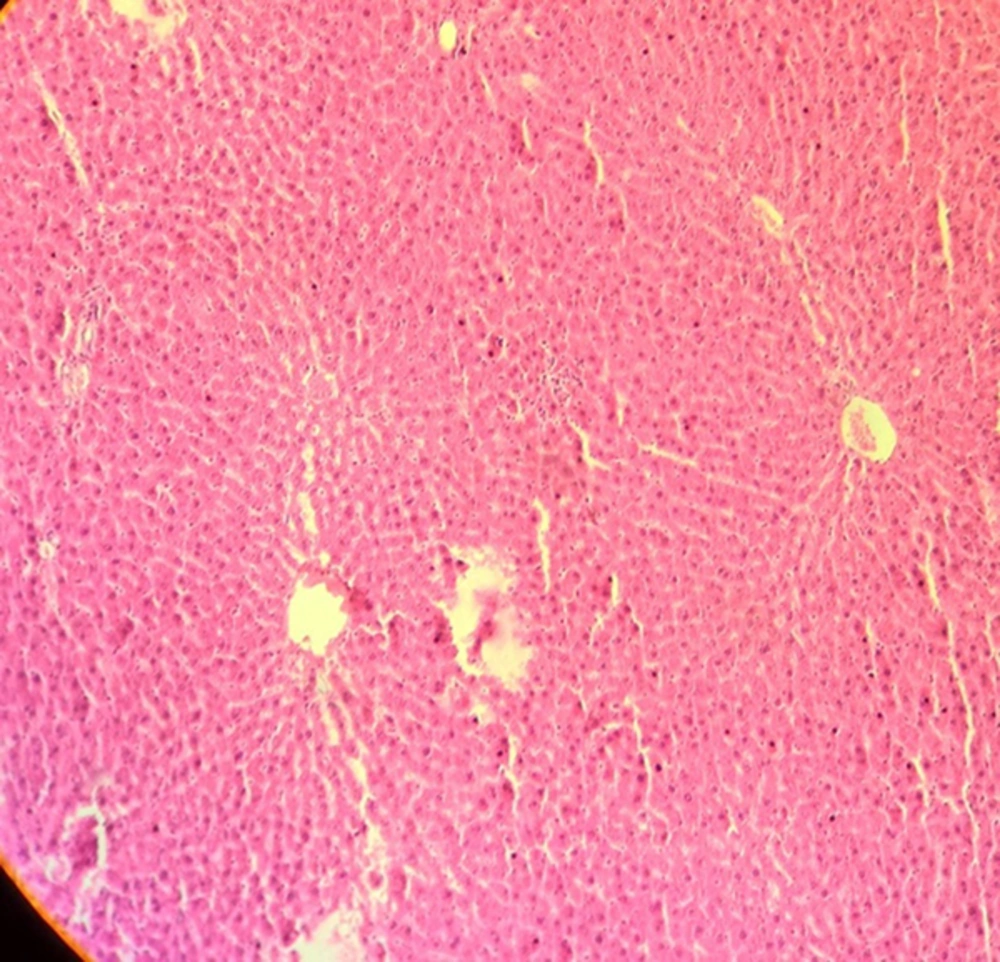
The results of light microscopy are brought in Figures 2 - 5. Figure 2 shows the images related to 1 mT exposure for 1 month; as it is observed, focal hepatocitolysis and mild to moderate portal inflammation is visible. In the case of 1 mT exposure for 2 weeks (Figure 3), apoptosis and hepatocyte inflammation has occurred. However, for the groups exposed to the lower field strength (0.5 mT), 1-month exposure (Figure 4) has caused hepatocyte focal inflammation, parenchymal hepatocitolysis and focal apoptosis. At this intensity, exposure for 2 weeks did not affect the liver tissue (Figure 5). Besides, in order to quantify our results, the chronic active hepatitis grading (25) was used, which results are brought in Table 1.
| Group | A | B | C | D | Total Score | Reversible/Irreversible |
|---|---|---|---|---|---|---|
| 1 mT-1 Month | 0 | 0 | 3 | 2 | 5 | R |
| 1 mT-2 Weeks | 0 | 0 | 2 | 0 | 2 | R |
| 0.5 mT-1 Month | 0 | 0 | 1 | 0 | 1 | R |
| 0.5 mT-2 Weeks | 0 | 0 | 0 | 0 | 0 | R |
aA, Interface hepatitis; B, Confluent necrosis; C, Spotty focal necrosis (hepatocitolysis); D, Portal inflammation.
4. Discussion
Nowadays, innovations in technology have resulted in changing lifestyle. This change has been concomitant with some new factors, which have the potential to affect mankind health. One of these factors are electromagnetic waves, which are present everywhere around us with different sources, frequencies, and intensities that have motivated researchers to work on the biological effects of such agents at different levels from cell to the in vivo conditions (1-3). The ICNIRP (26) has considered an occupational reference level of the magnetic flux density of 0.5 mT and the public reference level of 0.1 mT. We considered a magnetic flux density at the occupational level and twice it as 1 mT. Besides, we selected a fulltime worker’s time (all working days within a month) and a halftime worker (2 working weeks within a month). Our results showed that for the 0.5 mT magnetic flux density, the group exposed for 2 weeks showed no change in liver tissue relative to the control group, while at this magnetic flux density, 1 month of exposure caused formation of focal inflammation, parenchymal hepatocitolysis, and focal apoptosis (Figure 4, Figure 5, Table 1). At the higher magnetic flux density of 1 mT, the 2-week exposure leads to focal apoptosis and focal hepatocyte inflammation, which for 1-month exposure results to focal hepatocitolysis and mild to moderate portal inflammation. As it is observed, exposure at the occupational limit cannot lead to irreversible changes and its effects on cells are enough below moderate changes. However, in the case of 1 mT exposure, the effects are moderate and irreversible and they are spread in larger areas than the lower flux density of 0.5 mT. Again, the exposure time affects the level of stress on the cells and therefore, 1 month of exposure induces more stress than the 2 weeks of exposure. The microscopic trauma is correlated with the molecular changes at the cell level such as changes in oxidative stress indicators, metabolism of free radicals and superoxide dismutase (SOD) (27-32). Our findings are in line with Emre et al., where by measuring oxidative stress indicators suggested a relation between exposure to the magnetic field and cell death (28), or Martinez et al., who used higher flux densities and studied molecular indicators (32). Canseven et al., (27) studied effects of 1 mT magnetic flux density with different exposure times on guinea pigs tissues and concluded that exposure time affects free radical formation as we found via microscopic study. Hashish et al., (29) by studying the effect of whole body exposure of mice, showed that a relation between exposure to ELF-EMF and oxidative stress exists, which in our study, microscopic outcomes of this exposure were recorded. Also, Liu et al., (31) reported similar changes. Besides, Cakir et al., studied the effect of exposure to ELF-EMF at a flux density of 0.97 mT and reported no change in liver weight after exposure, which is in accordance with our study as we showed no fibrosis and therefore, we do not expect any changes in liver weight. On the other hand, Zecca et al., (33) reported no pathological changes in animal growth rate and also morphology and histology of the liver tissue, which is due to the small magnetic flux density they implemented in their study.
4.1. Conclusion
A microscopic study on the effects of ELF-EMF exposure was performed on rat liver tissue at the occupational exposure level and twice that for the exposure time similar to a halftime and a fulltime worker. It was observed that for a halftime exposure at occupational level, there exist no changes in liver tissue while for the fulltime exposure, some adverse effects were visible. Besides, at the higher magnetic flux density of 1 mT, both the halftime and fulltime exposure resulted in adverse irreversible cellular effects. By the way, it seems it is better to conduct the experiment on a wider period of time (maybe up to 3 - 6 month) to observe more severe effects and simultaneously measure the level of some enzymes effective on the function of the liver.