1. Background
Cancer is one of the main causes of death around the world (1). In recent years, there have been many studies conducted to investigate the application of nanomaterials in cancer diagnosis and treatment. Nanomaterials are small objects with at least one dimension of 1 - 100 nm (2). Nanomaterials can damage cell membranes and DNA and lead to cell death through reactive oxygen species (ROS) production; thus, they can destroy cancer cells (1, 3). Mesoporous silica nanoparticles (MSN) known as inorganic nanoparticles (4) are non-metal (5), solid, and highly porous oxides (6). These nanoparticles are used broadly in engineering, industry, biomedical applications, cosmetics, and FDA-approved food additives (7). MSNs are plentiful in nature, confirmed as generally recognized as safe (GRAS) by the FDA (8). One of the main advantages of MSN is explicitly low toxicity profile in vivo. Although clinical translation remains challenging, MSN is a promising tool for innovative, efficient, and safe cancer therapies (9-11). The fabrication of MSNs is simple and cost-effective (4). MSNs have the following desirable features as nano-vehicles: low toxicity, large pore volume, large surface area, ease of size-controlling, high synthesis scalability (12), low immunogenicity, and ability to be endocytosed by cells (13), tailorable pore sizes, and dual-function surfaces (exterior and interior) (14). MSNs as drug carriers have been used for cancer treatment on small creatures showing good results (15). One of the most important features of MSNs as carriers is controlled drug release (16, 17). One of the problems in cancer therapy is the resistance of cancer cells to the drugs that is called cancer multi-drug resistance (MDR). MSNs can deliver siRNA and lead to a reduction in drug resistance of cancer cells (18). Increasing the efficiency and reducing the side effects of anticancer drugs are the characteristics of MSNs (19, 20). MSNs can be used as drug delivery vehicles because of their biocompatibility, the absence of systemic toxicity, and specific targeting of tumor tissues through binding to antibodies or ligands (21-23).
HpD is a complex mixture of monomeric and aggregated porphyrins with a photosensitizing ability. By systemic administration, HpD accumulates in cancer cells; when activated with laser light at 630 nm (in presence of oxygen), it produces singlet oxygen and other reactive oxygen radicals that result in local radical-mediated cancer cell death (24).
The aim of this study was to evaluate the effects of HpD-loaded MSN on the cell proliferation and invasion in human breast adenocarcinoma cancer cell line, MCF7 (25). The antioxidant effects of this structure were also studied.
2. Methods
2.1. Cell Culture
Human breast cancer cell line, MCF7 (NCBI NO.C135), was purchased from Iran Pasteur institute. The cells were cultured in RPMI 1640 (Gibco, Invitrogen GmbH, Darmstadt, Germany) containing 10% fetal bovine serum (FBS) (Gibco, Invitrogen GmbH, Darmstadt, Germany), 100 unit/mL Penicillin and 100 µg/mL Streptomycin at 37°C in a humidified incubator with 5% CO2. This study was confirmed by the Ethics Committee of Semnan University of Medical Sciences.
2.2. Mesoporous Silica Nanoparticles (MSN)
MSNs were synthesized according to the available methods (26) and loaded with HpD to be used in the study.
2.3. Cell Assays
2.3.1. Cell Viability Assessment Using MTT Assay
To investigate the cell survival and proliferation, the MTT assay was performed. Cells were cultured in a flask; when the concentration reached 80%, the cells were trypsinized and moved to a 96-well plate. In each well, 5000 cells were cultured in 200 µL RPMI1640. After being cultured for 24 hours, the cells were exposed to MSNs alone and loaded with HpD. Different concentrations of nanoparticles as 0, 10, 20, 30, 40, and 50 µg/mL were used. After that, 10 µL of MTT reagent (0.5 mg/mL) was added to the cells on the day of testing. The contents of each well were replaced with 100 µL DMSO. Absorbance in each well was recorded at 570 nm with an Elisa reader system (Rayto software). Each experiment was done in triplicate.
2.3.2. Wound Healing Assay
A wound healing assay was performed to investigate the migration potential of MCF7. The MCF7 cells were cultured in 6-well plates. After 24 hours, the center of the wells was scraped with a sterile micropipette tip to create a straight scratch with the same width. Then, each well was washed with PBS solution, MCF7 cells were exposed to MSN and HpD-loaded MSN, and placed in an incubator for 48 hours. The nanoparticles concentration was in IC50 concentration. The wound closure was recorded with an inverted microscope.
2.4. Anti-Oxidant Assays
2.4.1. DPPH (2, 2-Diphenyl-1- Picrylhydrazyl) Assay
DPPH is a radical that absorbs light at 515 nm. In presence of an antioxidant, the radical reduces and absorbance diminishes. The antioxidant activities of MSN, HpD-loaded MSN, and vitamin C were measured.
2.4.2. FRAP (Ferric Reducing/Antioxidant Power) Assay
The FRAP (ferric reducing antioxidant power assay) assay is a method of wide suitability for the assay of antioxidants in vitro as well as in organisms. To carry out this reaction, the FRAP reagent was prepared. This reagent is a mixture of iron chloride (FeCl3) and TPTZ that were solved in a suitable buffer. The mixture has a yellow color. When a sample is added to this solution, the reagent reduces and a blue complex with Fe+2 appears. The maximum absorption capacity of this complex is at 593 nm. Ascorbic acid (1 mg/mL) was used as a standard antioxidant compound. The antioxidant activities of MSN, HpD-loaded MSN, and vitamin C were measured.
2.5. Statistical Analysis
Each assay was triplicated and data were obtained as Means. Statistical analyses were performed with SPSS statistical software (ver.17 SPSS/PC Inc., Chicago, IL, USA) and graphs were provided using Microsoft Excel 2007. After evaluation of normality and homogeneity of variables, the analysis of variance (ANOVA) was performed with a 95% confidence interval (P value ≤ 0.05); for multiple comparisons, Tukey test was used.
3. Results
3.1. Assessment of Cancer Cells Viability After Exposure to Silica Nanoparticles Loaded with HpD
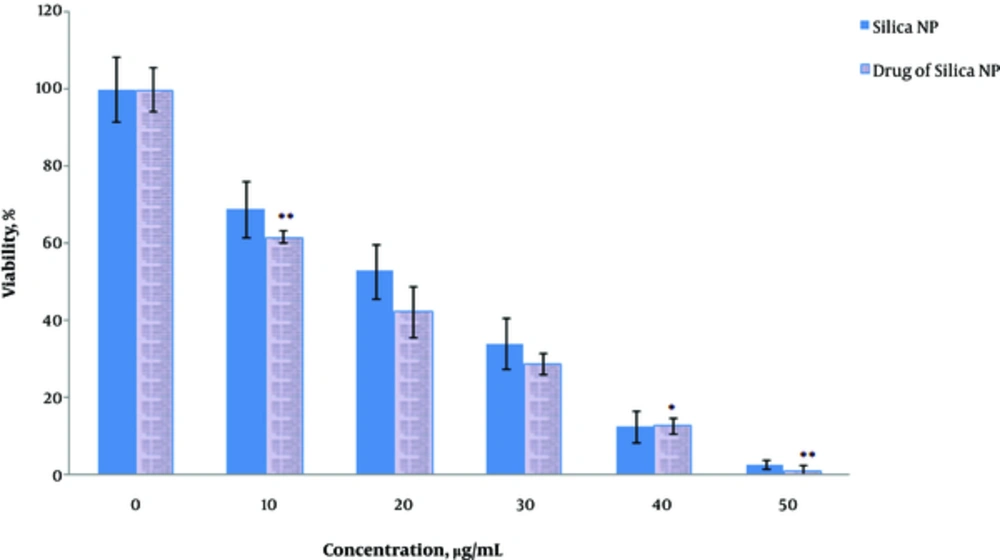
MCF7 cells were exposed to HpD-loaded MSN and the cell viability in 48 hours with different concentrations of the nanoparticles (0, 10, 20, 30, 40, and 50 µg/mL) was evaluated using the MTT assay (Figure 1). As nanoparticles concentration elevated, cell toxicity increased (P = 0.02). The most effective concentration was 50 µg/mL; in this concentration, the cell viability was the lowest (P = 0.01) (Figure 1).
3.2. The Decrease in the Invasion of Cancer Cells After Exposure to Silica Nanoparticles Loaded with HpD
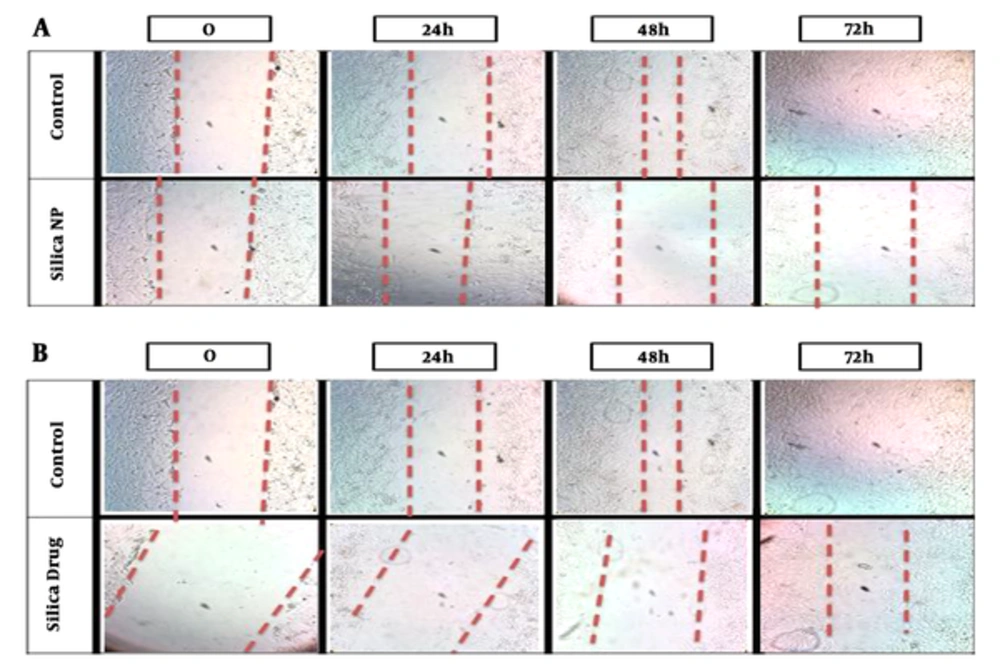
Invasion of cancer cells after exposure to nanoparticles was evaluated using the wound healing assay. There was a significant difference (P = 0.001) between the control group and the MSN alone and loaded with HpD groups (P value = 0.025) and migration of cancer cells significantly decreased (P = 0.04) (Figure 2).
3.3. Silica Nanoparticles Loaded with HpD Anti-Oxidant Activity
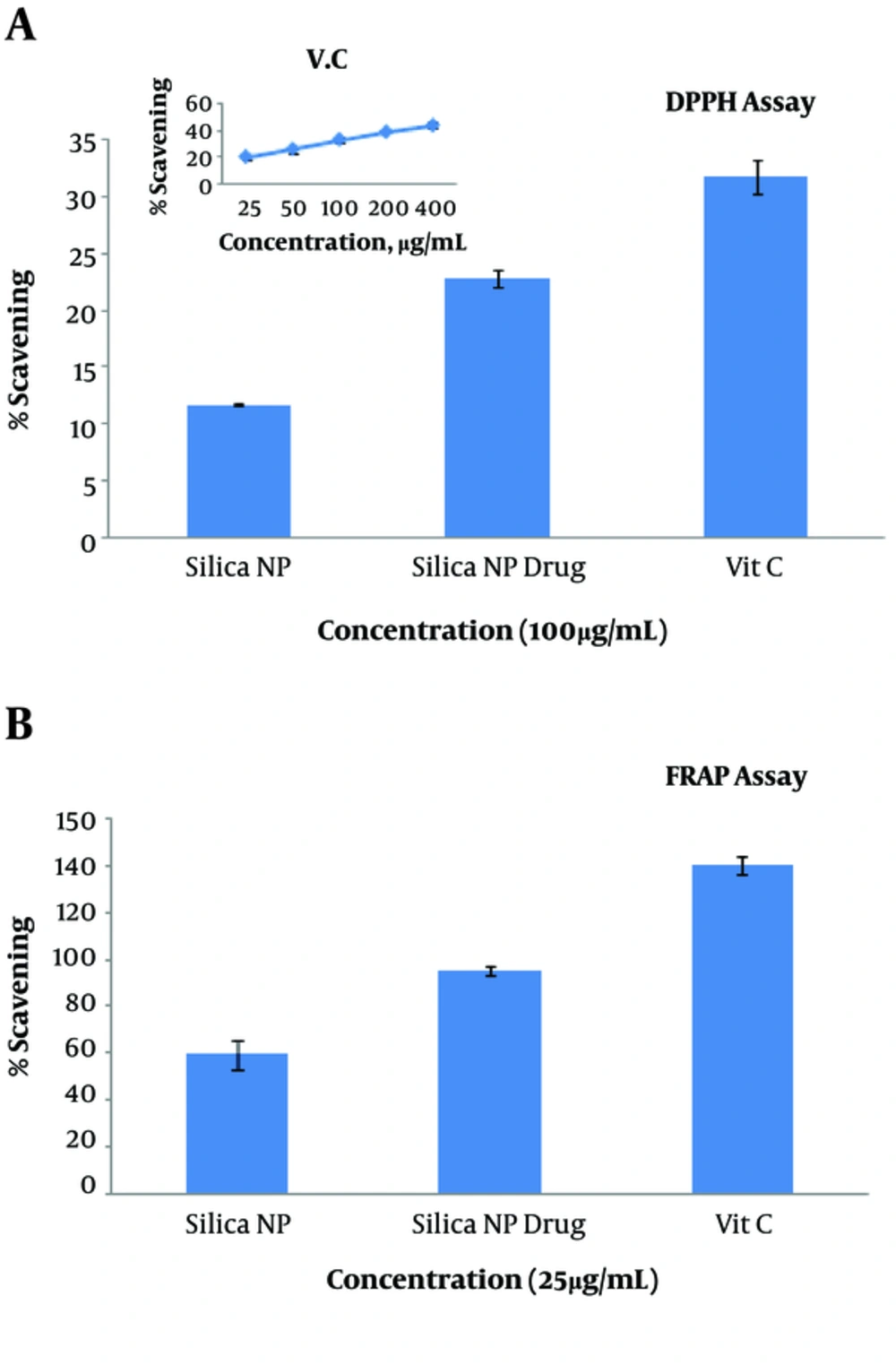
The antioxidant capacity of MSN alone and loaded with drug HpD was assessed and compared with that of vitamin C. Both MSN alone and loaded with drug HpD had an antioxidant ability, but their antioxidant capacity was lower than that of vitamin C (P = 0.031). MSN loaded with HpD had higher antioxidant ability than MSN alone (Figure 3).
4. Discussion
In this study, the effects of MSN loaded with HpD on MCF7 cell line were studied. When MCF7 cells were exposed to MSN loaded with HpD, the proliferation and viability of cancer cells decreased. Some studies showed that MSN could be used as drug carrier (15, 27). In this study, with increasing the concentration of MSN loaded with HpD, the inhibitory effects against cancer cells enhanced. In previous studies, it was shown that MSN increased ROS levels and reduced glutathione level (5, 28). In addition, these nanoparticles can cause membrane damage and lipid peroxidation. MSN accumulates in the cell nucleus, leads to DNA damage, and disturbs replication and transcription processes (29). All of these results show that MSN loaded with HpD causes cancer cells death. The antioxidant capacity of MSN loaded with HpD was also investigated. The results showed that MSN loaded with HpD had antioxidant capacity. The antioxidant effect of MSN is related to their biocompatibility (22, 30). In previous studies, it has been shown that MSN possesses biocompatibility features (21, 31). These results show that MSN without affecting healthy tissues circulates in the bloodstream and when reaches to the tumor cells, exerts its inhibitory effects.
The effects of MSN loaded with HpD on the invasion of cancer cells were evaluated. The results showed cancer cell invasion decreased. EMT (epithelial-mesenchymal transition) happens in the invasion (32). It seems that MSN inhibits the invasion through EMT inhibition.
This study was done in vitro. The investigation of these nanoparticles in vivo will provide a better understanding of their effects on normal and cancer cells. Altogether, in vivo studies for the experimental validation of MSN loaded with HpD are suggested.
4.1. Conclusion
In conclusion, our results showed that MSN loaded with HpD decreases the viability and proliferation of cancer cells in breast cancer cell line MCF7. Besides, these nanoparticles loaded with HpD reduce cancer cell invasion in vitro that should be studied in future by in vivo studies. The higher antioxidant capacity of MSN loaded with HpD compared to MSN alone makes it a promising compound to be used in animal models of cancer after being optimized through in vitro assays. This study indicates that MSN loaded with HpD leads to less invasive behavior and increased cell death. It seems that this structure affects cancer cells through ROS creation. This study shows that MSN loaded with HpD can provide a therapeutic strategy for cancer treatment. MSN had antioxidant capacity; according to these results, MSN can be used as a carrier for cancer drugs due to its biocompatibility. These nanoparticles can circulate in the bloodstream without any harm to healthy tissues and when reaching the tumor site, they can release drugs. This study suggests that MSN loaded with HpD is a stable, biocompatible, and effective agent for cancer treatment.