1. Background
Oral health in patients requiring critical care is vital because it can affect the health and clinical outcomes of these individuals (1, 2). Oral epithelium cells, extending from the lips to the oropharynx, are easily damaged in ICU patients due to poor circulation, lack of fluid and food intake, and toxicity of administered drugs (3). Oral microflora in ICU patients is not the same as in healthy individuals since it contains organisms that may rapidly give rise to pneumonia. Within 48 hours after admission, the composition of these patients’ microflora changes from Gram-positive streptococci to Gram-negative pathogens, most of which cause ventilator-associated pneumonia (VAP) (4). Guarantying oral hygiene and care is one of the main duties of health care providers and an essential component of ICU nursing care (5); thus ICU patients heavily depend on nurses to meet their nutrition and hygiene needs, including oral health. The research results show that oral hygiene deteriorates after a patient is admitted at the ICU (6, 7), and ICU nurses devote less attention and priority to this concern than they do to other typical measures performed in this ward (5). Moreover, there are a number of studies suggesting that nurses’ knowledge of this subject is not up-to-date (8-11). Teaching oral care to nursing students is underestimated and has not evolved considerably over the last 120 years (12). Owing to a variety of reasons such as fear of endotracheal tube displacement, pulmonary aspiration, patients’ increased distress and suffering, shortage of time, lack of sufficient knowledge and skills in oral care, unpleasantness of the task, and the perception that oral care is less important than other care procedures, nurses either completely ignore patients’ oral health or poorly attend to it (6). As part of nurses’ daily tasks, oral care in ICU patients is an effective method for lowering the incidence of VAP (1). Pulmonary infections are the most common nosocomial infections in ICU patients, and they account for 65% of all such infections in these individuals (13). Indeed, VAP is the second most widespread nosocomial infection (14, 15) and the first most prevalent infection and cause of death among respiratory infections in the ICU (16). This respiratory infection occurs 24 - 48 hours following endotracheal intubation and mechanical ventilation (17). One of the causes of VAP in ICU patients is dental plaque formation. The results of the study by Fourrier et al. on 57 ICU patients revealed a strong association between bacteria present in the dental plaque culture and those existing in the culture of tracheal secretions (18). Dental plaque can be removed both through toothbrush and antimicrobial agents like mouthwash (19). One of the methods of VAP prevention is to use chemical solutions with antibacterial properties; the most conventional of these solutions is chlorhexidine mouthwash. Chlorhexidine is a broad-spectrum antibiotic that is widely used by healthy people to prevent dental plaque formation and prevent or treat gingivitis (20). Although chlorhexidine has been proposed as the most potent anti-plaque agent (6, 21, 22), the Centers for Disease Control and Prevention has not yet approved it due to lack of enough evidence substantiating its efficacy (23). The World Health Organization has encouraged to conduct research to find natural substances such as plant extracts in order to overcome the side effects of medication such as antibiotic resistance (24).
Miswak (miswaak, siwak, sewak) is a tooth-cleaning stick from the Salvadora persica tree; it is also called Arak in Arabic and has been used for more than 7,000 years (25). Miswak is made from a compact set of tiny natural fibers (26); in addition to its antibacterial and antiseptic effects, it contains several natural chemical compounds that are essential for maintaining oral health (27). Apart from its antibacterial function which may help control the formation and progression of dental plaque, miswak can be used as a natural toothbrush and offer many medical benefits. Moreover, using miswak has also been emphasized in Islamic jurisprudence (28), and its effectiveness in improving the oral health of the general population has been established (29, 30).
Based on phytochemical analyses, some of the natural substances present in Salvadora persica include sodium chloride, calcium oxalate, silica, fluoride, sulfate compounds, tannic acid, and vitamin C. In addition to mechanical removal of bacterial plaque, miswak prevents the growth of dental plaque and oral microbes. It also has antimicrobial properties that stop the process of tooth decay and halt the development of many microorganisms. Moreover, WHO recommends the use of miswak as an effective tool for oral hygiene (28).
Oral hygiene practices vary across countries and cultures. Despite the widespread use of toothbrush and toothpaste, natural methods of cleaning teeth using a chewing stick from branches, stems, or roots of different plant species have been around for thousands of years in Asia, Africa, the Middle East, and the United States. If used properly, they can be as effective as conventional toothbrush in eliminating dental plaque thanks to their combined effect of mechanical cleaning and increasing saliva flow. Today, chewing stick is still common in many developing countries owing to factors such as religion or tradition, its availability, low cost, and ease of use. Also, WHO encourages using these methods. The 2000 Consensus Report on Oral Hygiene suggests that miswak, while its impact needs to be investigated further, could play a major role in promoting oral health (29). Administering chlorhexidine mouthwash in intensive care units could entail a number of complications such as tooth and mucosal discoloration, mucosal injury, formation of sialoliths, burning and dry mouth, and adverse systemic effects in case of swallowing (4).
2. Objectives
This clinical trial aimed at comparing the effect of oral care through miswak and chlorhexidine mouthwash on the incidence of VAP in patients admitted at the ICU of Khatam al-Anbia Hospital in Zahedan, Iran, in 2018.
3. Methods
This two-group pretest-posttest, single-blinded randomized clinical study was conducted in 2018 after obtaining the permission from the Vice-Chancellor for Research and Information Technology and the approval of the Ethics Committee of Zahedan University of Medical Sciences (IR.ZAUMS.REC.1397.447). The study population comprised qualified patients admitted at ICU of Khatam al-Anbia Hospital in Zahedan. The inclusion criteria consisted of anesthetized patients aged 18-65 years, insertion of endotracheal tube on admission to ICU and its maintenance during the study, lack of immunoi, hepatitis, or HIV infection, scoring below 11 based on Beck oral assessment scale, no history of herbal allergy, no hospitalization prior to admission to ICU, no history/symptom of gastric content aspiration, no coagulation disorders, no removable denture, at least 24 hours passed the admission to ICU, no pregnancy, no chronic pulmonary disease (including chronic obstructive pulmonary disease, lung cancer, and chest trauma), scoring below 5 based on the modified clinical pulmonary infection score at the onset of the study. On the other hand, the exclusion criteria were patient’s death, patient’s transfer to other departments before the end of the study, any visible oral injury and bleeding caused by endotracheal intubation or oropharyngeal airway insertion after the start of the study, removal of endotracheal tube for any reason, endotracheal re-intubation or tracheostomy at the time of the study, diagnosis of aspiration symptoms after the start of the study as documented in the patient’s admission records, restriction in oral care practices and thus risk of aspiration, developing pneumonia 48 hours after starting the study as diagnosed by the physician, withdrawal of mechanical ventilation before 96 hours, and requiring cardiopulmonary resuscitation.
Using the following formula and the incidence rate of VAP reported by Yao et al. (2011), the sample size was estimated at 16 for each group within the confidence interval of 95% and statistical power of 95%. In order to ensure sample size adequacy and to take account of possible attrition, 35 patients were allocated to each group (total = 70) (25).
P2 = 0.17
q2 = 0.83
P1 = 0.71
q1 = 0.29
Z1-α/2 = 1.64
Z1-β = 1.96
Data were gathered using a demographic and clinical information questionnaire, MCPIS, and BOAS (used to determine qualified subjects). The demographic questionnaire included information such as age, gender, history of smoking, cause of hospitalization, medical diagnosis, history of the underlying disease, as well as the type and dosage of antibiotic at the time of admission.
In fact, MCPIS is a standardized measure that has five criteria, including body temperature, pulmonary secretions, leukocyte count, PaO2/FiO2 ratio (mmHg), and chest radiography. A score of 0 - 2 is given to each criterion, and the maximum score of this instrument is 10. Scoring over 5 suggests the patient has developed VAP (31). Sabery et al. (32) confirmed the reliability of this tool based on Cronbach’s alpha and internal correlation coefficient (91%). Sensitivity and specificity of MCPIS have been reported to vary from 65 to 89.3% and from 58 to 100%, respectively (33). To ascertain the reliability of the examining physician, a pulmonologist observed all chest X-rays and confirmed the presence of pulmonary infiltrates.
Another scale was BOAS, which has 5 sub-scales (lips, gingiva and oral mucosa, teeth, tongue, and saliva), is scored on a 4-point Likert scale and scored 1 - 4. The total score of this scale ranges from 5 to 20. The lower the score is, the better the oral health of the patient will be (meaning no problem or disorder). Conversely, higher scores indicate more alarming degrees of disorder. Specifically, 5 means no disorder, 6 - 10 suggests mild disorder, 11 - 15 shows moderate disorder, and 16 - 20 represents severe disorder. Indeed, BOAS was used to enroll eligible patients. Cronbach’s alpha coefficient was 0.83 for this scale.
After obtaining the necessary permission to initiate the research, the researcher introduced herself to the head nurse and explained the purpose of the study. Next, once the study procedure was described, the informed consent was acquired from the companions of patients who met the inclusion criteria. Using convenience sampling, qualified patients were enrolled and then randomly divided into the intervention and control groups through coin flipping (heads = intervention group, tails = control group). For five consecutive days, oral care was provided twice a day (every 12 hours) using miswak in the intervention group and 0.2% chlorhexidine mouthwash in the control group. The oral care intervention was conducted as follows.
The patient was initially put in a proper position. In the absence of medical prohibition, the head of the bed was raised by 30 degrees; in case of medical prohibition, the patient was laid on one side and was supported by placing a pillow behind him/her and turning his/her head to one side. After washing her hands, the researcher wore gloves, glasses, and a mask to place the absorbent pad under the patient’s mouth. Once the number on the endotracheal tube and the endotracheal cuff pressure were checked, the patient’s mouth was opened and the airway, if any, was removed and cleaned. For oral care, the patient’s oral cavity was divided into four sections (top right, bottom right, top left, and bottom left). After that miswak was wetted using cool water, which had been boiled for 15 minutes, it was used to gently brush back and forth all interior, exterior, and masticatory surfaces of the teeth. In the control group, the oral care procedure was similar to the intervention group except that, instead of miswak, a cotton swab dipped in 0.2% chlorhexidine was administered. Next, in both groups, 20 cc of normal saline was poured into the oral cavity, the tracheal secretions were immediately suctioned, and the lips were cleaned. Eventually, the strip of the endotracheal tube was replaced and, using an applicator, a small amount of Vaseline jelly was applied to the lip surface and the patient was put back in his/her rest position. Before each intervention (miswak or chlorhexidine mouthwash), all areas of the mouth were examined by flashlight to identify coagulum, redness, ulcers, and bleeding. Individuals with a score of 11 or higher at any stage of oral health assessment were excluded. The patients in both groups were hospitalized for the first 48 hours and then examined daily by an anesthesiologist; if a patient developed pneumonia during the first 48 hours, he/she would be excluded from the study. In this research, no such instance took place.
Based on the five criteria of body temperature, pulmonary secretions, leukocyte count, PaO2/FiO2 ratio (mmHg), and chest radiography, MCPIS was completed for each patient during the first 12 hours before secretion sampling in order to diagnose possible pneumonia. The researcher filled out MCPIS again on the fifth day after the intervention. Finally, an intensivist confirmed or rejected pneumonia diagnoses. On the fifth day too, oral care was administered in both groups. To meet the blinding criterion, patients and the physician responsible for pneumonia diagnosis were not aware of the distribution of the two study groups.
Data were analyzed in SPSS 22 using chi-square test (to compare the two groups in terms of gender, cause of hospitalization, type of antibiotic used, smoking, and history of ICU hospitalization), independent t-test (to compare the two groups in terms of age, level of consciousness, and antibiotic dosage), and Fisher’s exact test (to compare the two groups in terms of VAP incidence). A P value of less than 0.05 was considered statistically significant.
4. Results
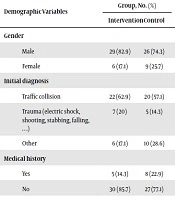
Finally, 70 intubated patients completed the study. There was no significant difference in the mean age between the miswak group (33.65 ± 13.50) and chlorhexidine group (34.83 ± 13.95) (P > 0.05). The mean Glasgow Coma Scale (GCS) of the patients in the miswak group was 5.8 ± 1.24 and 5.7 ± 1.36 in the chlorhexidine group, which were not significantly different (P > 0.05). Similarly, other demographic and disease information such as gender, cause of hospitalization, type of antibiotic used, smoking, and history of ICU admission did not differ significantly between the two groups (Table 1). None of the patients in the intervention and control groups had a history of smoking and ICU admission.
| Demographic Variables | Group, No. (%) | P Valuea | |
|---|---|---|---|
| Intervention | Control | ||
| Gender | 0.38 | ||
| Male | 29 (82.9) | 26 (74.3) | |
| Female | 6 (17.1) | 9 (25.7) | |
| Initial diagnosis | 0.49 | ||
| Traffic collision | 22 (62.9) | 20 (57.1) | |
| Trauma (electric shock, shooting, stabbing, falling, …) | 7 (20) | 5 (14.3) | |
| Other | 6 (17.1) | 10 (28.6) | |
| Medical history | 0.35 | ||
| Yes | 5 (14.3) | 8 (22.9) | |
| No | 30 (85.7) | 27 (77.1) | |
| Type of antibiotic | 0.97 | ||
| None | 7 (20) | 6 (17.1) | |
| Ceftriaxone | 13 (37.1) | 12 (34.3) | |
| Ceftriaxone + cefazolin | 9 (25.7) | 10 (28.6) | |
| Other antibiotics | 6 (17.1) | 7 (20) | |
aChi-square
The results showed that, after receiving oral care, none of the patients in the intervention group developed VAP, but 6 individuals (17.1%) in the control group were diagnosed with this condition. Fisher’s exact test pointed to a significant difference between the two groups with regard to VAP incidence (P = 0.01) (Table 2).
| VAP Incidence | Group, Frequency (%) | P Valuea | |
|---|---|---|---|
| Intervention | Control | ||
| Yes | 0 (0) | 6 (17.1) | 0.01 |
| No | 35 (100) | 29 (82.9) | |
| Total | 35 (100) | 35 (100) | |
aFisher’s exact test
5. Discussion
This study attempted to compare the impact of using miswak and chlorhexidine mouthwash for oral care on the incidence of ventilator-associated pneumonia in ICU patients. The results revealed that although none of the patients receiving miswak oral care developed VAP, some patients in the chlorhexidine group were diagnosed with VAP. Some of the studies conducted on the mechanical effects of miswak on the oral health of patients and healthy people are reviewed below.
Hafez et al. compared the effect of miswak as opposed to chlorhexidine + toothbrush on preventing VAP in intubated patients admitted to hospitals affiliated with Alexandria Faculty of Medicine, Egypt. The results displayed that dental plaque index significantly decreased in both groups. Moreover, a generally significant improvement occurred in the oral health of both groups. Oral health was not significantly different in the two groups on the day of admission and at the end of the study; however, the growth of microbial colonization was significantly lower in the miswak group than the other group. Furthermore, miswak postponed the emergence of VAP and, thus, reduced its incidence. Despite the longer duration of mechanical ventilation in the miswak group, the results suggested more favorable outcomes, including greater recovery and lower mortality. Additionally, colonization of teeth and oropharynx with potential respiratory pathogens was significantly lower in the miswak group than in the toothbrush + chlorhexidine group (34).
Hassane EL- Sol and Ahmad El-Gahsh investigated the effect of miswak versus routine oral care on the oral health of 56 ICU patients. Patients’ oral health was assessed three times before the intervention (oral care with miswak) as well as three and five days after the intervention. The results showed that patients’ oral health status on the third and fifth days after the intervention was favorable, and the authors concluded that miswak, thanks to its special antimicrobial properties, improves patients’ oral health and reduces infection (35). The results of this research are in line with those of the present study.
The results of the study by Haque and Alsareii illustrated that miswak (Salvadora persica) brings about beneficial effects in preventing oral diseases and promoting dental health. Miswak exerts an immediate impact on saliva composition, and several clinical studies have corroborated that the mechanical and chemical detergent effects of this chewing stick are equal and in some cases superior to conventional toothbrushes (36-38). In addition, exploring in vitro antimicrobial activity of Salvadora persica L. extract, Al-Bayati and Sulaiman (39) found that this species is active against all oral pathogens.
There are many studies into the effect of miswak extract and chlorhexidine as well as other mouthwash solutions on the incidence of VAP and oral hygiene in ICU patients. Some of these reports have obtained results compatible with the present study, while some others have suggested findings contradictory to our research (40-42). Moeintaghavi et al. addressed in vitro antimicrobial activity of miswak extract, chlorhexidine, and persica mouthwash against major oral pathogens, and the results indicated that chlorhexidine involves more effective antibacterial activity than persica mouthwash and miswak at all tested concentrations. The results of the above study also substantiated the argument that chlorhexidine is still the best antimicrobial agent and herbal mouthwashes have marginal antimicrobial activity. Researchers accentuate that mechanical plaque removal is the most significant method for preventing periodontal disease and that mouthwash is used to heighten its efficacy (43), which is consistent with the findings of the present study concerning the effect of chlorhexidine on reducing VAP incidence. However, our results also suggested that VAP incidence decreases much more sharply by miswak than chlorhexidine solution, which is probably due to the special mechanical and chemical properties of this teeth cleaning twig. In fact, our results depicted that both mechanical and chemical factors present in miswak helped prevent dental plaque formation in the intervention group. As mentioned above, miswak has been recommended as an oral care tool thanks to its chemical properties and the mechanical ability of its fibers to move between the teeth (37). On the other hand, oral care with swabs dipped in 0.2% chlorhexidine solution reduces plaque formation as a result of chemical processes (23). In a scanning electron microscopic (SEM) study, Almas (44) investigated the effect of miswak extract and chlorhexidine gluconate in human subjects and found that 0.2% chlorhexidine and 50% miswak extract had similar effects on dentin, even though 50% miswak eliminated a larger smear layer. The author also called for exploring the effect of miswak extract on diseased as well as sensitive teeth (45). Similarly, Akpata and Akinrimisi (46) studied the antibacterial activity of some extracts from African chewing sticks, and they observed that the positive effect of these extracts is primarily due to mechanical cleansing. It was also suggested that African chewing sticks, as a disinfectant, can control the formation and activity of dental plaques, hence reducing the incidence of periodontal disease and, possibly, tooth decay (45). These results are in good agreement with those of the current study.
Considering the widespread usage of miswak in the Middle East, Hardie and Ahmed examined the impact of this chewing stick on oral health and concluded that the fibrous branches of Salvadora persica produce mechanical effects and release beneficial chemicals (45), which is consistent with our findings despite the fact that Hardie and Ahmed studied a healthy population. Falahinia et al. studied oral care through 0.2% chlorhexidine solution + soft toothbrush and compared it with applying swabs dipped in 0.2% chlorhexidine solution. It turned out that using toothbrush alongside chlorhexidine solution could not reduce the incidence of VAP as dramatically as did the other procedure (47), which contradicts the results of the present research. This inconsistency could be because of the greater impact of miswak, compared to conventional toothbrush, on improving patients’ oral health. In this regard, one can point to the study by Al-Otaibi et al. who compared the effect of conventional toothbrush and miswak on dental plaque and gingival health; they reasoned that miswak could lessen plaque formation and enhance Gingival Index much more significantly than do conventional toothbrushes (48).
Several factors such as medical diagnosis, patients’ immune system, resistant pathogenic agents in the environment, airway suctioning, gavaging, and staff’s hand hygiene affect VAP incidence in anesthetized patients. One of the limitations of this study was lack of control over these factors; two others concern its low sample size and short duration. It is proposed that this study be pursued in a larger sample size until the subjects are discharged from the ICU or other specialized care units.
5.1. Conclusions
The results demonstrated that miswak can substantially decrease the incidence of ventilator-associated pneumonia in patients with endotracheal tube. Given its special properties, including its chemical effects (antioxidant, analgesic, anti-inflammatory, antimicrobial, and antineoplastic), mechanical effects (cleansing, antiplaque, and anti-calculus), simple and safe usage, and its affordability compared to other solutions and methods, it is highly recommended that this chewing stick be provided as part of oral care to ICU patients.