1. Background
Traumatic brain injury (TBI) is referred to damage to the brain that causes physical, mental, emotional, social, and occupational changes. It is a major cause of death and disability worldwide (1). The prevalence of these injuries in developed countries is 200 per 100,000 people (2). About one and a half million Americans suffer from head injury annually, of which around 230,000 people are hospitalized (3). In Iran, following cardiovascular diseases, traffic collisions are the second leading cause of death at different ages, and the first leading cause of death in people under 40 years of age (4). The most important site of injury in these individuals is the head, which often results in hospitalization and death (5). Coma is one of the main complications of brain injury (6). It refers to the state of not waking up, not responding without opening the eyes, and not being able to speak and obey commands. People who are in a coma are alive but unable to move and respond to their environment (7). Coma is associated with motor and cognitive dysfunction and leads to several life-threatening complications such as respiratory failure, pneumonia, pressure ulcers, and [pulmonary] aspiration (8). Mechanical ventilation in ICU is a key component in caring for patients with critical conditions (9). Although it is a life-sustaining treatment, it encounters patients with a variety of physical and psychological stresses, all of which result in their agitation (10). On the other hand, the environment of ICUs, because of their noise, light, and other stimuli, can often stir up agitation in patients (11). Agitation is a state of strong and violent emotions, sudden and intense movements and unpredictable behaviors, and a lack of awareness of time, place, and other people (12). It might cause actions such as intense and constant shaking, messing up the bed, and pulling tubes (13).
Wacker and Haley (14) found that patients requiring mechanical ventilation at ICU admission experience high levels of agitation. Tan et al. (15) showed that 93% of patients in the ICU experience agitation. Agitation in mechanically ventilated patients is associated with problems such as a prolonged stay in the ICU, increased duration of mechanical ventilation, and unpredictable tracheal disconnection (16), catheter disconnection, increased oxygen demand, and impaired therapeutic interventions (17). It also brings about other adverse outcomes such as excessive use of sedatives, increased costs, mortality, and the possibility of harming oneself and caregivers (18). Pharmaceutical methods and physical inhibitors are commonly prescribed in the ICU to control agitation (19). Evidence shows that administering restrictive devices for mechanically ventilated patients is not an appropriate measure and could pose more problems (20). The most common method to control agitation in these patients is the use of sedatives. Nevertheless, it could give rise to adverse effects such as delirium, decreased consciousness, changes in mechanical ventilation, inability to maintain and protect the airway, cardiovascular instability, prolonged dependence on mechanical ventilation, and ventilator-associated pneumonia (14). In Iran, to control agitation in ICUs, sedatives and analgesics are usually prescribed in the form of continuous infusion. Nurses often perform sedative injections and analgesics without following a protocol or using an instrument to measure patients’ calmness and agitation. This approach reduces the possibility of managing and controlling patients’ agitation. It is also possible that infusion might continue even if there is no need for these drugs or the patient’s required dose changes, both of which could expose the patient to the side effects of excessive sedation (21). An increasing number of studies in recent years have encouraged the use of non-pharmaceutical methods of relieving agitation. These cover a wide range of methods that are relatively simple, low-cost, and non-invasive and involve fewer complications than pharmaceutical methods (22). In this regard, the use of complementary techniques such as aromatherapy, massage therapy, music therapy, and touch therapy can offer many benefits (23). Touching serves as the body language and has been introduced as one of the most effective means of non-verbal communication; it is a behavior that determines intimacy and shows the need for love, dependence, and belonging (24). One of its main benefits is general relaxation (25). O’Lynn and Krautscheid (26) described touching as an essential component of patient care and a method of relaxation. On the other hand, hearing is the most important sense for understanding peace and security and the last sense that disappears in comatose patients; unlike other senses, there is no obstacle to stimulate this sense (27). Grap et al. (28) found that auditory stimulation affects the management of agitation. Administering the right sedation is one of the essential roles of nurses in relation to mechanically ventilated patients admitted to ICU (29).
Considering the need for sensory stimulation in these patients and give their complete dependency, in addition to the fact that nurses might not have the time and energy to give sensory stimulation, it seems that the presence of a family member in the ICU can be a good alternative for meeting this requirement (30). Naderi et al. (8) reported that it is much more effective if patients with decreased consciousness are provided with sensory stimulation by a family member. Encouraging the family to participate in sensory stimulation, besides providing them with the opportunity to get engaged in patient care, accelerates the improvement of patients’ cognitive status and disease prognosis (30).
2. Objectives
In this regard, the purpose of the present study was to determine the effect of auditory and tactile stimulation by family members on the level of agitation of TBI patients with decreased consciousness who were hospitalized in the ICUs of two educational hospitals.
3. Methods
This quasi-experimental study was conducted on TBI patients with decreased consciousness who had been admitted to a number of ICUs in southeastern Iran in 2019. A total of 80 eligible patients were selected through convenience sampling and then randomly assigned to the intervention (n = 40) and control (n = 40) groups.
The inclusion criteria for patients were: age of 18 years and older, passage of 24 - 48 hours of stabilization of hemodynamic symptoms, artificial ventilation, consciousness level of 5 - 8 based on the FOUR scale, and an agitation rate of +2 to +4 based on the Richmond Agitation and Sedation Scale (RASS), lack of underlying diseases such as diabetes (caused by possible neuropathies based on patient history), and lack of hearing impairment, skin disorder in the area of tactile stimulation, and neuromuscular and sensory-motor disorders. The inclusion criteria for the family member were: being a first-degree member of the patient’s family (father, mother, spouse, child, and sibling), the regular presence of a certain family member during the intervention, and emotional stability. On the other hand, the exclusion criteria were the patient’s death or transfer to other centers, instability of the patient’s hemodynamic status during the study, and emergency surgery due to increased intracranial pressure.
On the basis of the study by Nobahar et al. (31), the following formula, a confidence interval of 95%, and a power of 95%, we allocated 31 individuals to each group. However, in order to consider possible attrition, we finally considered 40 patients for each group (total = 80).
Data collection tools included a demographic form (age, gender, marital status, ethnicity, and history of ICU hospitalization) and the RASS. The latter covers indicators such as attention to the patient’s mood and degree of combativeness, the degree and type of movements of the limbs in terms of how purposeful they are and how much they execute commands, the degree to which patients endanger themselves and others, the state of consciousness and drowsiness as well as responding to commands, and finally, the patient’s level of agitation and arousability. RASS is a 10-point continuum from -5 to +4 classified into three levels. In this tool, the five negative points show different levels of sedation (-1 = drowsy, -2 = light sedation, -3 = mild sedation, -4 = deep sedation, and -5 = unarousable), zero denotes normal and calm behavior, and the four positive points show different levels of anxiety or agitation (+1 = restless, +2 = agitated, +3 = very agitated, +4 = combative). This scale was developed by Sessler et al. (17) at the University of Arkansas, and its validity and reliability have been confirmed. It was translated to Persian, and its content validity was assessed by Tadrisi et al. (32). The reliability of RASS was also reported by examining the interclass correlation coefficient (ICC) (0.95) (32). Yeganeh et al. (33) confirmed the reliability of RASS based on the internal consistency method and agreement among the evaluators (0.95). In the present study, inter-rater reliability was determined based on assessing 20 mechanically ventilated patients by two people (researcher and patient’s nurse), and the reliability coefficient was estimated at 0.94 using Spearman’s ρ.
Before referring to the ICUs to do the research, we obtained the necessary permits from the Vice-chancellor for Research and Information Technology and the Ethics Committee of Zahedan University of Medical Sciences; we also received a letter of introduction and presented it to the hospital officials so as to acquire their approval. Convenience sampling was used; thus, the researcher recruited any TBI patient with decreased consciousness and under mechanical ventilation who had been admitted to the ICU and who met the inclusion criteria. Written informed consent was obtained from the family of eligible patients who expressed their willingness to participate in the study. Then, they were fully informed about the objectives and process of the research. Subsequently, the selected patients were randomly divided into the control and intervention groups. Initially, 80 envelopes containing the name of a group (40 envelopes for the intervention and 40 others for the control) were prepared and then randomly arranged. As patients were gradually admitted and recruited, they were given a card in order, which determined their respective groups.
If a patient was assigned to the intervention group, the researcher held a meeting with his/her family members and asked them to introduce someone who had the most contact with and dependence on the patient and had no psychological problems (The person appointed by the family was fixed during the study). Then, the selected family members received the necessary training on how to wear gowns and footwear, wash hands, and perform auditory stimulation, which included introducing themselves to their patient, call their name three times, inform them of the time and place, and tell them happy memories near both their ears. Additionally, tactile stimulation included palpation of the patient’s wrist and palm from the wrist to the nail. All instructions were offered in practice, and the intervention was performed after coordination with the head nurse and nurse. Five minutes before the intervention, the demographic form was completed, and the patient’s agitation was measured using RASS. Next, under the supervision of the researcher, the family member performed the intervention based on the instructions, which included simultaneous auditory and tactile stimulation, first on one side, and then on the other side for 10 minutes. Agitation was re-measured 30 minutes after stimulation. The intervention was carried out for seven consecutive days in the evening shift (16:00 - 18:00 o’clock) due to its non-interference with the usual activities of the ward.
The demographic form was completed for patients in the control group, and then their agitation was measured using RASS but without doing auditory and tactile stimulation. This was done for seven days between 16:00 and 18:00 o’clock at intervals, similar to the intervention group. The collected data were analyzed in SPSS 21 using independent t-test, paired t-test, analysis of covariance, and chi-square test. P < 0.05 was considered statistically significant.
4. Results
The results of the Shapiro-Wilk Test showed that the data have a normal distribution; hence, parametric tests were used. It was found that the mean (and standard deviation) age in the intervention and control groups was 40.22 ± 14.3807 and 36.40 ± 11.80, respectively, implying no significant difference between the two groups in terms of age. As shown in Table 1, there was no significant difference between the two groups with regard to other personal and disease-related variables.
| Variable | Control | Intervention | P Value | ||
|---|---|---|---|---|---|
| Frequency | Percentage | Frequency | Percentage | ||
| Gender | 0.64* | ||||
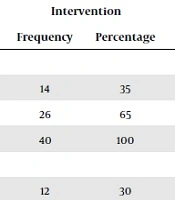
| Female | 16 | 40 | 14 | 35 | |
| Male | 24 | 60 | 26 | 65 | |
| Total | 40 | 100 | 40 | 100 | |
| Marital status | 0.21* | ||||
| Single | 3 | 7.5 | 12 | 30 | |
| Married | 37 | 92.5 | 28 | 70 | |
| Total | 40 | 100 | 40 | 100 | |
| Type of brain injury | 0.85* | ||||
| Bleeding | 13 | 32.5 | 13 | 32.5 | |
| Contusion | 18 | 45 | 16 | 40 | |
| Other traumas | 9 | 22.5 | 11 | 27.5 | |
| Total | 40 | 100 | 40 | 100 | |
| Device alarm | 0.09* | ||||
| Mild | 17 | 42.5 | 10 | 25 | |
| Moderate | 23 | 57.5 | 30 | 75 | |
| Total | 40 | 100 | 40 | 100 | |
The results indicated that the mean score of agitation on the first and second days before the intervention (P = 0.32, P = 0.42) and after the intervention (P = 0.32, P = 0.25) did not differ significantly in the two groups; however, this variation became statistically significant between the third and the seventh days before and after the intervention (Table 2). Analysis of covariance was employed to control this significant effect before the intervention in the two groups.
| Day/Time | Before Intervention, Mean ± SD | After Intervention, Mean ± SD | Mean Changes, Mean ± SD | P Valuea |
|---|---|---|---|---|
| 1st day | ||||
| Intervention | -3.92 ± 0.79 | -3.90 ± 0.81 | 0.02 ± 0.15 | 0.32 |
| Control | -4.07 ± 0.52 | -4.05 ± 0.50 | 0.02 ± 0.15 | 0.33 |
| P valueb | 0.32 | 0.32 | 0.18 | |
| 2nd day | ||||
| Intervention | -3.90 ± 0.81 | -3.85 ± 0.76 | 0.05 ± 0.22 | 0.16 |
| Control | -4.02 ± 0.57 | -4.02 ± 0.57 | 0.0 ± 0.0 | 0.18 |
| P valueb | 0.42 | 0.25 | 0.15 | |
| 3rd day | ||||
| Intervention | -3.72 ± 0.84 | -3.62 ± 0.89 | 0.10 ± 0.30 | 0.44 |
| Control | -4.30 ± 0.60 | -4.30 ± 0.60 | 0.0 ± 0.22 | 0.23 |
| P valueb | 0.001 | 0.001 | 0.09 | |
| 4th day | ||||
| Intervention | -3.37 ± 0.89 | -3.25 ± 0.83 | 0.12 ± 0.33 | 0.32 |
| Control | -4.32 ± 0.65 | -4.27 ± 0.71 | 0.05 ± 0.31 | 0.23 |
| P valueb | 0.001 | 0.001 | 0.3 | |
| 5th day | ||||
| Intervention | 4.0 ± 30.85 | 4.0 ± 27.84 | 0.0 ± 0.15 | 0.32 |
| Control | 3.0 ± 15.94 | 3.0 ± 15.94 | 0.0 ± 0.0 | 0.23 |
| P valueb | 0.001 | 0.001 | 0.31 | |
| 6th day | ||||
| Intervention | -3.15 ± 0.94 | -3.15 ± 0.94 | 0.0 ± 0.0 | 0.30 |
| Control | -4.30 ± 0.85 | -4.27 ± 0.84 | 0.02 ± 0.15 | 0.32 |
| P valueb | 0.001 | 0.001 | 0.32 | |
| 7th day | ||||
| Intervention | -3.10 ± 0.98 | -3.02 ± 0.94 | 0.07 ± 0.26 | 0.83 |
| Control | -4.32 ± 0.85 | -4.32 ± 0.85 | 0.0 ± 0.0 | 0.61 |
| P valueb | 0.001 | 0.001 | 0.07 |
aPaired t-test
bIndependent t-test
The necessary conditions for using analysis of covariance were fulfilled on the basis of the results of Levene’s test (which suggested the normality and consistency of variances) as well as regression homogeneity (which indicated the absence of a significant interaction between the independent variable and the associated variable). The results of the analysis of covariance demonstrated that the mean score of agitation on the third, fourth, and fifth days after the intervention was not significantly different in the two groups (P = 0.25, P = 0.5, P = 0.08), which means that auditory and tactile stimulation could not significantly reduce the mean score of agitation on these days.
Meanwhile, the two groups experienced significantly different levels of agitation on the sixth and seventh days after the intervention (P = 0.001, P = 0.01), such that auditory and tactile stimulation significantly reduced agitation in the intervention group.
5. Discussion
The mean score of agitation in the intervention and control groups was assessed before and after tactile and auditory stimulation for seven days, and no significant variation occurred in the two groups in terms of this score between the first and the fifth days after the intervention. Sosnowski and Ustik (34) highlighted the time factor in performing sensory intervention and concluded that assessing the trend of changes in ICU patients over time is more helpful than their examination at some specific points in time. This is because patients’ agitation score, under the influence of various factors, changes over time with decreasing consciousness. It seems that more than seven days are required for sensory stimulation to produce its effects. Both Urbenjaphol et al. (35) and Moghadam and Payami Bousari (36) studied the effects of sensory stimulation for 14 days, which is twice as long as our study period.
The results of our study showed that the mean score of agitation in the two groups was significantly different 6 and 7 days after tactile and auditory stimulation. This could be due to starting the intervention in the first 24 - 48 hours after the patient’s hemodynamic symptoms were stabilized. Indeed, conducting appropriate early interventions to stimulate TBI patients with decreased consciousness reduces coma complications, and evidence suggests that the highest rate of brain reset takes place during the first weeks of brain injury, and initiating an early sensory stimulation program can facilitate the healing process (37).
Our findings are consistent with those of Nobahar et al. (31), Moattari et al. (38), and Souri Lakie et al. (39). Thus, Moattari et al. (38) reported that sensory stimulation (tactile and auditory) by family members, as opposed to nurses, is more effective on the level of cognitive function and the rate of basic cognitive, sensory recovery in comatose patients with severe TBI. The remarkable feature of this study was in performing all five types of sensory stimulation, whereas we limited our intervention to just tactile and auditory stimulation. Souri Lakie et al. (39) reported that touching improved both respiratory status and arterial blood saturation in agitated patients undergoing mechanical ventilation. The similarity of this study with ours is in tactile stimulation, which is only one of the two types of stimulation that we targeted. Nobahar et al. (31) observed that touching the patient’s wrist under mechanical ventilation led to a significant change in the level of agitation. This study dealt only with tactile stimulation, but we simultaneously considered two kinds of stimulation.
In a different line, Kavei et al. (40) concluded that foot reflexology in reflex points of the heart and lungs could not significantly relieve anxiety and agitation in mechanically ventilated patients after heart surgery. This inconsistency in the results can be attributed to differences in the nature of the interventions and the study subjects under study.
Touching has been proposed as a safe, effective, practical, and simple intervention for relieving patients’ agitation (41) and reducing pain and anxiety (42) with no harmful complications (43). Heiderscheit et al. (11) found that auditory stimulation via music is effective in lowering anxiety and agitation in patients with decreased consciousness.
Naderi et al. (8) similarly stated that sensory stimulation has a positive impact on patients with decreased consciousness, and it will be much more efficient if stimulations are performed by a family member. In addition to receiving care from the treatment team, ICU patients need peace of mind, comfort, and support, which can only be provided with the family and other people close to the patient (44). Accordingly, patients’ families are not just visitors, but they act as caregivers and partners in the treatment team, especially when making decisions (45). Hasanzadeh et al. (46) stressed that the sensory stimulation of comatose patients by family members is an integral part of care that is overlooked in ICUs. As one of the resources available to perform sensory stimulations, patients’ family members can voluntarily spend their time and energy for the recovery of their loved ones. Therefore, revising the rules banning people from visiting their patients in ICUs can provide a suitable platform for exploiting the positive effects of the presence of a family member on the patient’s bedside. Failure to implement the intervention for more than seven days, inability to control unwanted sensory stimuli in the environment, and the provision of sensory stimulation by a family member with different qualities were the limitations of this study.
5.1. Conclusion
According to the results of this study, sensory stimulation reduces the agitation of TBI patients with decreased consciousness. Therefore, any kind of care and intervention that helps lower the level of agitation in these individuals can improve the prognosis of the disease. In this regard, it is recommended that necessary education should be given to nurses and family members to provide comatose patients with purposeful stimulation of all five senses. Targeted sensory stimulation is an easy and cost-effective technique, which can be adopted to alleviate the agitation of TBI patients hospitalized in ICUs.
Since ICU nurses do not have enough time to perform sensory stimulation, it is practical to seek help from the patient’s family, thereby involving them in the care process. In addition to helping toward realizing a family-based care system, this cooperation reduces both the patient’s agitation and the family’s concerns and anxieties.