1. Background
Ostomy is a surgical procedure that creates an opening in the body for the excretion of body wastes (1). In Iran, the number of ostomies is about 30,000; of them, 70% are colostomy, 20% ileostomy, and 10% urostomy. Over 2,000 patients are members of the Iranian Ostomy Society. Of these patients, about 10% have temporary problems, and the rest has permanent problems who undergo operation each year due to different reasons (2). It was reported that in Iran, more than 70% of ostomy patients experienced at least one complication related to ostomy surgery over the first two months after surgery (3). The most common complications were wounds, irritation, and inflammation in the peristomal skin around the ostomy due to the skin’s contact with the urine or stool excreted from the stoma. Another common complication was the gradual destruction of the epidermis around the stoma due to the repetitive installation and removal of the ostomy pouch (4-6). Persistent wounds and inflammation of the skin around the ostomy can reduce the patients’ quality of life and increase their hospitalization and medical costs (1, 6, 7).
There are several strategies for peristomal skincare. These strategies include methods related to the pouch, such as an accurate cutting method for ostomy pouch, gentle removal of the used pouch from the skin, and the use of alternative types of pouches, as well as the application of hydrocortisone ointments, medicinal herbs, commercial gels, and other skin-care products (8, 9) Pittman et al. (10), indicated that 50% of the patients suffered from peristomal skin complications, including leakage, peristomal moisture-associated dermatitis, and stoma pain. These complications may remain even after treatment with skin-care products and procedures. In addition, Colwell et al. (11), reported that treatment with commercial gels can significantly increase the cost of stoma care and lead to skin irritation and inflammation due to the application of gel removers. Furthermore, other complications, such as allergic reactions, contact dermatitis, and an increase of sweating and skin moisture, have been reported as the results of gel therapy (6). It has also been reported that the treatment of the skin around the stoma using topical steroids is associated with skin atrophy, wound-healing disorders, and an increase in sweating and telangiectasia (11-13).
Chamomile is a type of herb that is used in traditional medicine in Iran (14) Srivastava et al. (15), reported that the chamomile extract had various medical benefits, including anti-bacterial, anti-inflammatory, anti-convulsive, and disinfectant benefits. These benefits are due to the presence of certain compounds, such as chamazulene and alpha-bisabolol, in chamomile extract (15). Several studies on animal and human subjects have investigated the effects of chamomile extract on surgery sites and burn wounds (16-18) Charousaei et al. (17), compared the effects of chamomile extract and hydrocortisone 1% ointment for the treatment of inflamed skin around the stoma. Their results showed that chamomile extract reduced inflammation in a shorter time and with fewer complications compared to prednisolone ointment (17). There is lacked studies regarding the effects of chamomile extract on frequent peristomal skin problems following ostomy surgery.
2. Objectives
The present study was conducted to investigate the effect of chamomile extract on the prevention of peristomal skin complications, including wounds, secretion, and color change.
3. Methods
3.1. Research Design
This single-blinded, placebo-controlled, randomized clinical trial was conducted on 80 ostomy patients who were referred to Koroush Health Clinic, Isfahan, Iran, in 2018. The study population consisted of ostomy patients who underwent colostomy or ileostomy in the previous month.
3.2. Participants
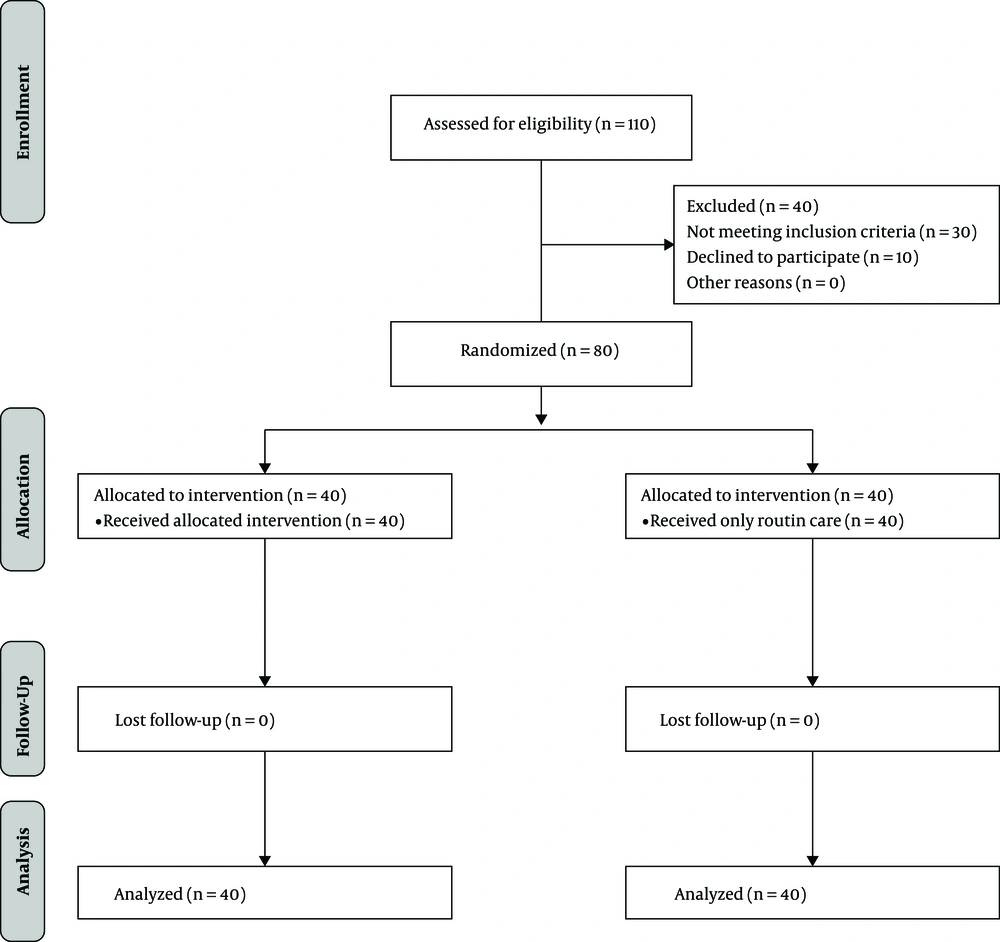
The sample size was estimated using the Pocock sample size formula (19). According to Azhari et al. (16), d (the difference between the two mean scores) and ∂ (standard deviation) were 7.8 and 7, respectively, with a type I error probability of 0.05 and a power of 0.80 Accordingly, the optimal sample size in each group was estimated at 40. Participants were randomly assigned into intervention and control groups using Random Allocation Software (simple allocation using www.random.org). First, 110 patients were assessed for their eligibility to participate. Thirty patients did not meet the inclusion criteria. Ten patients declined to participate. A total of 80 participants were randomly assigned to intervention (n = 40) and control (n = 40) groups based on the block randomization method (Figure 1).
The inclusion criteria for this study included an age range of 18 to 60 years, having a colostomy or ileostomy in the previous month, a lack of secretion and inflammation in the skin around the stoma at the beginning of the study, and a lack of chemotherapy or radiotherapy over the last four months. Patients with skin disease history, such as lupus, psoriasis, skin allergies, and dermatitis, were excluded from this study.
3.3. Instrument
The study instrument consisted of a demographic information questionnaire, including gender, occupation, education level, surgery type, surgery reason, ostomy type, radiotherapy history, and chemotherapy history, and the ostomy skin tool (OST). The OST was developed by the present study’s authors for assessing peristomal skin. The OST is a checklist that was developed for measuring the peristomal skin in a simple, efficient, and reliable method. To complete the OST checklist, the patient’s peristomal skin needs to be assessed by a healthcare professional.
The OST includes three attributes related to abnormal changes in peristomal skin. The items/attributes include color change, secretion, and wound. The color change attribute includes normal, pink, and red categories. The secretion attribute includes categories of no secretion, serous secretion, sanies secretion, and infectious secretion. Categories related to the wound consist of no wound and wound. The validity of the OST was confirmed by 10 faculty members who were experts in wound management at Isfahan University of Medical Sciences.
3.4. Ethical Considerations
The study protocol was approved by the Ethics Committee of Kashan University of Medical Sciences (Research number: 1397.019) and was registered in the Iranian Registry of Clinical Trials (Registration code: IRCT 20180802040674N1). Written consent was obtained from the participants after explaining the purposes of the study.
3.5. Interventions
Before the intervention, sociodemographic and clinical information questionnaires were completed by the participants. Furthermore, the first author, who is a wound and ostomy expert, evaluated the peristomal skin in terms of color change, secretion, and the existence of the wound to complete the OST. For participants in both groups, the same ostomy bags (Coloplast Alterna) in the appropriate sizes were used. The participants and/or their caregivers were trained to replace the ostomy bag when needed. They were trained to perform peristomal skin routine care, including washing the area with water after removal of the old bag.
In the intervention group, in addition to the routine care, the participants applied five drops of 12% hydroglycolic extract of chamomile on the skin around the stoma. They applied the drops with cotton or gauze swabs. After the skin dried, they positioned a new ostomy bag in place. Instead of the chamomile extract, the participants in the control group were provided with distilled water as a placebo. For blinding purposes, chamomile extract and distilled water (placebo) were provided in similar containers with even and odd codes. Accordingly, chamomile extract containers were labeled with even codes, and distilled water containers were labeled with odd codes. Other steps regarding skin and ostomy care were similar in both intervention and control groups. At the beginning of the study, the first author performed skin and ostomy care in both groups to train the participants and/or their caregivers and ensure their ability to complete skin and ostomy care over the course of the study. Participants in both groups were instructed to inform the research team of possible changes in peristomal skin or ostomy care, such as the use of other solutions.
The chamomile extract was provided by the same company (Kashan Barij Essence Company). The first author examined the peristomal skin in the intervention group using the OST checklist once a week for four weeks. In the control group, this evaluation was performed at the beginning and the end of the study. The weekly evaluation in the intervention group was performed to assess the peristomal skin for any undue complications associated with chamomile extract. If a participant did not refer to the clinic at the scheduled time (once a week), the participant was given a reminder by the first author, and then a different time in the same week was scheduled. If the participant was not able to refer to the clinic, the author visited the participant at the participant’s convenient time and place. In case of peristomal wounds or secretions in the intervention group, the use of chamomile was stopped, and the participants were asked to continue the routine ostomy care and refer to visit their physicians.
After the intervention, chamomile extract was introduced to the control group participants. In the intervention group, the use of chamomile extract continued based on their physicians’ opinions. The intervention and control groups were compared in terms of OST scores at the beginning and the end of the study.
3.6. Statistical Analysis
The data were analyzed using SPSS software, version 16. Continuous demographic variables were reported with the mean and standard deviation, and categorical demographic variables were reported with frequencies and percentages. A chi-square test was used to analyze data related to gender, occupation, education level, surgery type, surgery reason, ostomy type, radiotherapy history, and chemotherapy history. The chi-square and Mann–Whitney tests were used to compare the two groups in terms of the peristomal wound, secretion, and color change. The level of significance was set at 0.05.
4. Results
The results indicated that about 57.5% of the participants in the intervention group and 55% in the control group were males. Most participants in the intervention group (70%) and the control group (77.5%) had a temporary ostomy. In both groups, the most common reason for the ostomy surgery was colon cancer (75%). In the intervention group, about 35% and 67.5% had a history of radiotherapy and chemotherapy, respectively. However, in the control group, 32.5% and 52.5% had a history of radiotherapy and chemotherapy, respectively. There was no significant difference between the intervention and control groups in terms of sociodemographic and clinical information (Table 1).
| Variable | Experimental Group | Control Group | Chi-Square Test (χ2) | P- Value | ||
|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | |||
| Age (y) | 0.60 | 0.06 | ||||
| 18 - 40 | 15 | 37.5 | 13 | 32.5 | ||
| 40 - 60 | 25 | 62.5 | 27 | 67.5 | ||
| Gender | 0.05 | 0.82 | ||||
| Female | 18 | 45 | 17 | 42.5 | ||
| Male | 22 | 55 | 23 | 57.5 | ||
| Occupation | 0.73 | 0.87 | ||||
| Retired | 13 | 32.5 | 13 | 32.5 | ||
| Housewife | 14 | 35 | 11 | 27.5 | ||
| Employee | 13 | 32.5 | 16 | 40 | ||
| Educational level | 0.52 | 0.06a | ||||
| Elementary school | 17 | 42.5 | 14 | 35 | ||
| Middle school | 13 | 32.5 | 14 | 35 | ||
| High school | 3 | 7.5 | 7 | 17.5 | ||
| University | 7 | 17.5 | 5 | 12.5 | ||
| Marital status | 0.60 | 0.05 | ||||
| Unmarried | 5 | 12.5 | 4 | 10 | ||
| Married | 35 | 87.5 | 36 | 90 | ||
| Surgery type | 0.58 | 0.45 | ||||
| Permanent ostomy | 28 | 70 | 31 | 77.5 | ||
| Temporary ostomy | 12 | 30 | 9 | 22.5 | ||
| Surgery reason | 0.20 | 0.90 | ||||
| Colon cancer | 30 | 75 | 30 | 75 | ||
| Inflammation of the intestine | 5 | 12.5 | 6 | 15 | ||
| Ileus | 5 | 12.5 | 4 | 10 | ||
| Ostomy type | 0.05 | 0.82 | ||||
| Colostomy | 15 | 37.5 | 16 | 40 | ||
| Ileostomy | 25 | 62.5 | 24 | 60 | ||
| Radiotherapy history | 14 | 35 | 13 | 32.5 | 0.06 | 0.81 |
| Chemotherapy history | 27 | 67.5 | 21 | 52.5 | 1.87 | 0.17 |
a Mann–Whitney test
The Chi-Square test showed that after the intervention, the frequency of peristomal skin wound was significantly lower in the intervention group than in the control group (χ2 = 9.04, P = 0.003). Furthermore, the results of the Mann-Whitney test showed that at the end of the study, peristomal skin secretion (χ2 = 5.47, P < 0.001) and peristomal skin color change (χ2 = 5.51, P < 0.001) were significantly lower in the intervention group than in the control group (Table 2).
| Variable | Experimental Group | Control Group | Chi-Square Test (χ2) | P- Value | ||
|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | |||
| Wound | 9.04 | 0.003a | ||||
| No | 37 | 92.5 | 26 | 65 | ||
| Yes | 3 | 7.5 | 14 | 35 | ||
| Secretion | 5.47 | < 0.001b | ||||
| No | 35 | 87.5 | 9 | 22.5 | ||
| Serous secretion | 2 | 5 | 13 | 32.5 | ||
| Sanies secretion | 2 | 5 | 15 | 37.5 | ||
| Infectious secretion | 1 | 2.5 | 3 | 7.5 | ||
| Color change | 5.51 | < 0.001b | ||||
| No changes | 34 | 85 | 9 | 22.5 | ||
| Pink | 3 | 7.5 | 10 | 25 | ||
| Red | 3 | 7.5 | 21 | 52.5 | ||
aChi-square test was used for these groups
b Mann–Whitney test was used for these groups
5. Discussion
Our results showed the effect of chamomile extract on preventing peristomal skin complications. After the intervention, the peristomal skin complications, including wound, secretion, and color change, were significantly lower in the intervention group than in the control group. Multiple studies have addressed the effects of herbal medicinal interventions, such as the use of chamomile extract, and recommended these interventions to be used in addition to conventional treatments for the management of diseases and complications related to diseases (20-23).
Charousaei et al. compared the effect of chamomile extract and hydrocortisone ointment 1% on peristomal skin lesions in colostomy patients (17). Their results showed that peristomal skin lesions were significantly lower in the chamomile group than in the control group. Other research teams examined the effects of chamomile extract on skin problems associated with children’s diaper rash (24, 25). Afshari et al. indicated that chamomile ointment was more effective in healing children’s diaper rash and dermatitis than calendula ointment (24). However, Ghanipour Badelbuu et al. did not find any significant difference between chamomile extract and aloe Vera gel in preventing diaper rash and dermatitis among children (25).
Chamomile is an effective herbal medicine in alternative and complementary medicine. It has been used for different purposes, such as the management of dermatological, gastrointestinal, neurological, and psychiatric problems (26). Several clinical studies have evaluated the effectiveness of chamomile in wound healing (27-29). Glowania et al. (1987) showed that chamomile extract and chamazulene were effective in healing the wounds associated with new tattoos (27).
Martins (2009) asserted that herbal medicines, such as chamomile, have lower rates of adverse effects, are more convenient and easy to use, and are less expensive. Martins (2019) recommended the use of unconventional treatments for the management of diseases and complications associated with diseases and conventional treatments (30). Our study showed the effectiveness of the chamomile extract on the prevention of peristomal skin complications among our participants. With the increasing number of patients with chronic diseases, it is essential to study and introduce innovative strategies for the management of diseases and their associated complications.
5.1. Limitations, Strengths, and Recommendations
A strength of this study was the use of placebo and blinding methods. This study had limitations. The generalizability of our findings is limited due to the small sample size. We could not control some confounding variables, such as nutritional and lifestyle factors, which could influence the results of the study. We recommend further studies with a larger sample size. Furthermore, in future research, study designs to exclude or control confounding variables, such as general health, lifestyle, and nutritional factors, would be valuable.
5.2. Conclusion
The complications associated with chronic diseases and their conventional treatments necessitate innovative interventions to help patients cope with their health conditions. In this study, we found that the chamomile extract was effective in preventing peristomal skin complications among the participants. We recommend the use of chamomile extract for the prevention of peristomal skin complications. To verify and integrate the results of the current study for evidence-based practice, further studies are recommended.