1. Background
Pregnancy is one of the most challenging psychological events in women’s life that can have positive and negative effects in the long run (1). During this period, the woman’s body undergoes anatomical and physiological changes for fetal growth, metabolic needs, and readiness for delivery (2). Pregnant women frequently experience physiological and metabolic changes that affect different systems in their body organs (3). Since the mother’s cardiovascular, pulmonary, immune, and metabolic systems are altered during pregnancy, maladjustment of the mother’s physiology during this period may cause abnormal birth weight or gestational diabetes mellitus (GDM) (4).
GDM is defined as the degree of blood sugar that is a metabolic disorder with first recognition during pregnancy and includes type 2 diabetes that has not been previously diagnosed and GDM that develops later in pregnancy (5). The World Health Organization (WHO) defines GDM as glucose intolerance of variable degree (6). Blood sugar is normal if the fasting blood sugar level is less than 92 and the blood sugar two hours after the meal is less than 153, but if any of them is equal to or higher than these levels, it is considered GDM (7), which is diagnosed in the second or third trimester (8).
GDM is currently the most common complication of pregnancy (5). However, its prevalence varies in different parts of the world, and the possible causes of this variation are unknown with no precise spatial classification. Nevertheless, the available data indicate that GDM is prevalent in 14% of pregnancies worldwide, accounting for approximately 18 million annual births (9). According to the estimations, 16.9% of all pregnancies worldwide (10) and 17% of all pregnancies in Iran are associated with GDM (11). Diabetes in the sensitive period of pregnancy has profound effects on the internal environment of the uterus and may impair fetal or maternal function (12).
One of the most serious complications of GDM is a decrease in sexual function. This metabolic disorder not only can cause neurovascular injury, but also have psychological damages (13). The prevalence of sexual dysfunction in people with diabetes is about three times higher than in the general population (14). The WHO defines sexual health as a state of physical, emotional, mental, and social well-being in relation to sexuality (15). Sexual function plays an essential role in marital satisfaction. Thus, people who have satisfying sexual relations have a higher quality of life and do not fail to express love and affection to their spouse, and have more understanding and empathy in life (16). As a result, if the sexual function is impaired, quality of life and marital satisfaction are affected.
Sexual dysfunction in women is demonstrated as sexual disorders including hypoactive sexual desire disorder (HSDD), which itself involves the absence of sexual fantasies, sexual arousal disorders, orgasmic disorders, sexual pain disorders (dyspareunia), general medical disorders, substance use disorders, and unclassified sexual disorders (17, 18). Although sexual desires are innate and involuntary, sexual attitudes and behaviors can be learned. Therefore, sexual activities can have different meanings for different people and change from time to time. Accordingly, by increasing awareness, the person acquires the necessary ability to solve sexual problems (19). This awareness is gained by increased education on sexual issues.
Education is a process that starts with informing, is followed by creating motivation, and ultimately leads to improved behavior and performance (20). Health literacy is one of the most important determinants of health in the world (21), practicing health-promoting behaviors is one of the best ways to stay healthy (22), and participation is a key value for health promotion (23). Health-promoting behaviors include activities that enable people to take control of their health (22).
2. Objectives
Since pregnancy care and screening programs are very important, especially in high-risk pregnancies, and as few interventional studies have addressed mental and sexual health in women with GDM, the present study aimed to examine the effect of a sexual health promotion training program on the sexual function of pregnant women with GDM referring to comprehensive health centers in Zahedan, Iran in 2020.
3. Methods
This quasi-experimental study was conducted using a pretest-posttest design with two groups. The participants were 80 pregnant women with GDM referring to comprehensive health centers in Zahedan to receive prenatal care in 2020. The inclusion criteria were women in the age range of 18 - 40 years, gestational age of 30 - 30 weeks, the absence of physical illnesses affecting sexual function or known mental disorders, normal pregnancy, no history of pre-GDM, diagnosis of GDM based on routine tests performed according to national guidelines, no use of medications affecting glucose metabolism, the absence of sex ban, and obtaining a score of 28 or less on the Female Sexual Function Index (FSFI). The exclusion criteria were the occurrence of fetal and maternal complications or any traumatic accident during the course of study and absence or non-participation in more than one training session.
The sample size in this study was estimated as 17 individuals per group using the mean and standard deviation for sexual function in a similar study (Moradi et al., 2016) with 95% confidence interval (CI), 95% statistical test power, and based on the following formula. Given that the participants were selected using multi-stage sampling, the estimated sample size was multiplied by two. Thus, the number of participants in each group was 34 individuals. To ensure the sample adequacy and due to the possible dropouts, the sample size for each group was estimated as 40 individuals (total sample size: 80 individuals). To this end, clinics and health care centers were selected using a multi-stage method, and then the participants in each center were selected in proportion to the assigned sample size using convenience sampling (24):
Z1 – α/2 = 26.59;
Z1 – β =22.48;
The data in this study were collected using two instruments: (1) the demographic information questionnaire assessed parental age; (2) the number of pregnancies, type of previous deliveries, parental occupation, parental education, body mass index (BMI), and ethnicity. Besides, the FSFI was completed by the participants as a self-report instrument.
3.1. Female Sexual Function Index (FSFI)
This index was developed by Rosen et al. (2000) to measure female sexual function. It contains 19 items that measure sexual function in six independent domains: (1) desire (2 items), (2) arousal (4 items), (3) lubrication (4 items), (4) orgasm (3 items), (5) satisfaction (3 items), and (6) pain (3 items). The respondents are asked to choose the best option that shows their sexual function during the last four weeks. The answers are scored on a five-point Likert scale ranging from 0 to 4 (0 = never, 1 = rarely, 2 = occasionally, 3 = frequently, or 4 = almost always). To normalize the domains, the scores of each domain are added together and then multiplied by the corresponding factor load shown in Table 1. After the domains are normalized, the minimum and maximum scores for each domain are 1 and 6, respectively. The minimum and maximum scores for the whole index are obtained, and the sum of the scores for the six domains are equal to 2 and 36, respectively. Higher scores indicate better sexual functions in each domain and overall (25). The reliability and validity of the FSFI in Iran were assessed and confirmed by Mohammadi et al. (2008). To determine the validity of the index, its content validity was assessed using the content index of the instrument. The reliability of the instrument was evaluated and confirmed by calculating the Cronbach’s alpha coefficient, and the corresponding value was 0.87 (26). The reliability of the instrument in the present study was checked by measuring its internal consistency for the whole index, and the Cronbach’s alpha was 0.72.
| Domains | Number of items | Range | Factor load | Min | Max |
|---|---|---|---|---|---|
| Desire | 2 | 1 - 5 | 0.6 | 1.2 | 6 |
| Arousal | 4 | 0 - 5 | 0.3 | 0 | 6 |
| Lubrication | 4 | 0 - 5 | 0.3 | 0 | 6 |
| Orgasm | 3 | 0 - 5 | 0.4 | 0 | 6 |
| Satisfaction | 3 | 0 - 5 or 1 - 5 | 0.4 | 0.8 | 6 |
| Pain | 3 | 0 - 5 | 0.4 | 0 | 6 |
| Total | 19 | - | - | 2 | 36 |
After obtaining an ethics code from the Ethics Committee of Zahedan University of Medical Sciences and obtaining permission from officials of the University and affiliated health centers, the participants were selected using multi-stage sampling. Since Zahedan has five districts, we selected two centers from each district by drawing lots. To this end, the city was first divided into five northern, southern, eastern, western, and central districts, and two health care centers were selected randomly from each district (10 centers in total). From the two selected centers in each district, one center was allocated to the intervention group and one center to the control group. Then, in each center, eight women who met the inclusion criteria were selected using convenience sampling. Next, a list of all pregnant women with GDM in each center was prepared using the data from the electronic systems of selected health centers, and they were contacted by phone. Necessary information was given to them about the objectives of the study. If they agreed to participate in the study, they were enrolled in the study after obtaining a written informed consent. Before the intervention, the participants in the intervention and control groups completed the two questionnaires. Moreover, the FSFI was administered again in both groups four weeks after the completion of the sexual health promotion training program.
Given the COVID-19 outbreak, the pregnant women in the intervention group were divided into small groups, each with 4 to 5 members. Then, they were invited to participate in the counseling sessions by telephone. The location of the training program was chosen upon the participants' agreement, and four sexual health promotion counseling training sessions (two 60- to 90-minute sessions per week) were held in the clinic or the School of Nursing and Midwifery in Zahedan by maintaining social distancing and observing health protocols such as wearing masks and gloves due to the coronavirus disease 2019 (COVID-19) pandemic. The instructions provided in the training sessions are presented in Table 2. To answer the possible questions and address the concerns of the participants in the intervention group after the intervention, a direct telephone line was provided to them. Four weeks after the completion of the intervention, the participants in both groups were asked to complete the FSFI. During this period, the participants in the control group received only routine training and care in the centers.
| Session | The Instructional Content |
|---|---|
| 1 | An overview of the anatomy and physiology of the male and female reproductive system and its changes during pregnancy in simple language and based on participants’ perceptions, psychological changes in different stages of pregnancy, sexual response cycle in men and women, changes in the sexual response cycle in pregnancy, common sexual misconceptions, and challenging them through group discussions. |
| 2 | Exploring the attitudes towards sexual activity in pregnancy, a review of pregnancy-specific sexual disorders, the role of sex in marital relationships, and demonstrating effective and safe situations for sexual activity in pregnancy. |
| 3 | Managing pregnancy stress by practicing and applying stress reduction techniques such as imaging and progressive muscle relaxation, listening to music, managing emotions and using problem-focused coping mechanisms, and training to manage anxiety-related thoughts through distraction and positive self-talk. |
| 4 | Providing information on effective health care and lifestyles to cope with GMD by reviewing GMD and its complications, permitted physical activity training, proper diet training, self-care training, and psychological aspects of GMD, including worries and stress and their impact on women’s mental and sexual health. |
The collected and coded data were analyzed using SPSS software (version 24). To this end, the data were summarized using descriptive statistics such as frequency (percentage), mean (± SD), minimum, and maximum. To compare the pre- and post-intervention mean scores in each group and between the groups, the paired samples t-test, and independent samples t-test were used, respectively. Moreover, to compare the frequency of the qualitative variables in the two groups, the Monte Carlo, Fisher, and chi-square tests were used. Analysis of covariance (ANCOVA) was also run to determine the effectiveness of the intervention and control the effect of the pretest. All statistical procedures were performed at the significance level of less than 0.05 (P < 0.05).
4. Results
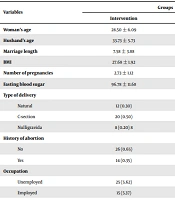
Given the number of participants in each of the intervention and control groups, the data from 40 pregnant women with GDM were analyzed. As shown in Table 3, the participants and their husbands in the intervention and control groups were in their 20s and 30s, respectively. Moreover, the participants in both groups had married on average for more than seven years. The mean fasting blood sugar of the participants in both groups was within the normal controlled range. The results of the independent samples t-test showed that there was no significant difference between the two groups in terms of age and marriage length. Besides, the results of the chi-square and Mont Carlo tests showed no significant differences in terms of other qualitative variables (P < 0.05).
| Variables | Groups | P-Value | |
|---|---|---|---|
| Intervention | Control | ||
| Woman’s age | 28.50 ± 6.09 | 28.98 ± 5.03 | 0.77 |
| Husband’s age | 33.73 ± 5.73 | 34.08 ± 4.98 | 0.77 |
| Marriage length | 7.58 ± 3.88 | 7.30 ± 2.91 | 0.72 |
| BMI | 27.68 ± 1.92 | 27.54 ± 2.19 | 0.86 |
| Number of pregnancies | 2.73 ± 1.12 | 2.85 ± 0.86 | 0.99 |
| Fasting blood sugar | 96.78 ± 11.68 | 101.18 ± 14.73 | 0.07 |
| Type of delivery | 0.11 | ||
| Natural | 12 (0.30) | 12 (0.30) | |
| C-section | 20 (0.50) | 26 (0.65) | |
| Nulligravida | 8 (0.20) 8 | 2 (0.5) | |
| History of abortion | 0.64 | ||
| No | 26 (0.65) | 24 (0.60) | |
| Yes | 14 (0.35) | 16 (0.40) | |
| Occupation | 0.47 | ||
| Unemployed | 25 (5.62) | 28 (0.70) | |
| Employed | 15 (5.37) | 12 (0.30) | |
| Education | 0.98 | ||
| Middle school | 12 (0.30) | 22 (0.55) | |
| Secondary school | 23 (5.57) | 14 (0.35) | |
| Higher education | 5 (5.12) | 4 (0.10) | |
| Husband’s education | 0.21 | ||
| Illiterate | 2 (0.5) | 3 (5.7) | |
| Middle school | 9 (5.22) | 15 (5.37) | |
| Secondary school | 12 (0.30) | 5 (5.12) | |
| Higher education | 17 (5.42) | 17 (5.42) | |
| Type of pregnancy | 0.39 | ||
| Planned | 34 (0.85) | 31 (5.77) | |
| Unplanned | 6 (0.15) | 9 (5.22) | |
| Husband’s occupation | 0.49 | ||
| Unemployed | 0 (0.0) 0 | 2 (5) | |
| Employed | 40 (100) | 38 (95) | |
a Values are expressed as mean ± SD or No. (%).
Table 4 shows the sexual function scores for the pregnant women with GDM in the intervention and control groups before the intervention (3.09 ± 0.86 and 2.86 ± 72, respectively) and after the intervention (3.75 ± 0.93 and 2.98 ± 0.55, respectively). The results of the independent samples t-test indicated no significant difference between the two groups in sexual function and its domains before the intervention (P > 0.05). However, significant differences were found between the two groups in sexual function and its domains after the intervention (P <0.05). The results of the paired samples t-test showed that the scores for sexual function and its domains (except for satisfaction and desire) significantly increased for the participants in the two groups after the intervention compared to the pre-intervention stage (P < 0.05). Given the significant differences in the mean scores of sexual function between the two groups before the intervention, the results of ANCOVA to control the significant effect of the pre-intervention scores showed that the mean scores of sexual function were significantly higher for the participants in the intervention group compared to the control group (P = 0.001) (Table 5).
| Variables | Stage | Changes | Paired Samples t-test | |
|---|---|---|---|---|
| Pre-intervention | Post-intervention | |||
| Desire | ||||
| Intervention | 3.09 ± 0.86 | 3.75 ± 0.93 | 0.66 ± 0.67 | 0.001 |
| Control | 2.86 ± 0.72 | 2.98 ± 0.55 | 0.12 ± 0.64 | 0.024 |
| Independent samples t-test | P = 0.21 | P = 0.001 | P = 0.001 | |
| Arousal | ||||
| Intervention | 2.62 ± 0.97 | 3.24 ± 0.62 | 0.62 ± 0.43 | 0.001 |
| Control | 2.16 ± 0.50 | 2.40 ± 0.62 | 0.24 ± 0.49 | 0.004 |
| Independent samples t-test | P = 0.009 | P = 0.001 | P = 0.001 | |
| Lubrication | ||||
| Intervention | 3.09 ± 0.94 | 3.72 ± 0.62 | 0.36 ± 0.56 | 0.001 |
| Control | 2.54 ± 0.73 | 2.71 ± 0.56 | 0.17 ± 0.47 | 0.02 |
| Independent samples t-test | P = 0.005 | P = 0.001 | P = 0.001 | |
| Orgasm | ||||
| Intervention | 3.03 ± 0.82 | 4.04 ± 0.63 | 0.99 ± 0.60 | 0.001 |
| Control | 2.67 ± 0.95 | 2.95 ± 0.66 | 0.28 ± 0.85 | 0.04 |
| Independent samples t-test | P = 0.07 | P = 0.001 | P = 0.001 | |
| Satisfaction | ||||
| Intervention | 3.04 ± 0.85 | 4.21 ± 0.67 | 1.17 ± 0.54 | 0.001 |
| Control | 2.78 ± 1.02 | 2.81 ± 0.79 | 0.03 ± 0.61 | 0.76 |
| Independent samples t-test | P =0.22 | P = 0.001 | P = 0.001 | |
| Pain | ||||
| Intervention | 2.78 ± 0.82 | 3.94 ± 0.59 | 1.16 ± 0.63 | 0.001 |
| Control | 2.61 ± 0.93 | 2.92 ± 0.75 | 0.13 ± 0.82 | 0.02 |
| Independent samples t-test | P = 0.39 | P = 0.001 | P = 0.001 | |
| Total score | ||||
| Intervention | 17.65 ± 4.41 | 22.89 ± 3.23 | 5.24 ± 2.23 | 0.001 |
| Control | 15.62 ± 3.79 | 16.78 ± 3.16 | 1.15 ± 3.13 | 0.02 |
| Independent samples t-test | P = 0.03 | P = 0.001 | P = 0.001 | |
a Values are expressed as mean ± SD unless otherwise indicated.
| Source | Sum of Squares | df | Mean Square | F | Sig. | Effect Size | Power |
|---|---|---|---|---|---|---|---|
| Pretest | 450.42 | 1 | 450.42 | 99.29 | 0.001 | 0.65 | 1 |
| Group | 457.92 | 1 | 457.92 | 100.94 | 0.001 | 0.65 | 1 |
| Error | 349.29 | 77 | 4.53 | ||||
| Total | 33029.70 | 80 |
5. Discussion
The data in this study showed that the sexual health promotion training program had a positive and significant effect on the overall sexual function of the pregnant women with GDM, so that sexual function and its domains were significantly improved after the intervention in the intervention group compared to the control group. To demonstrate the effectiveness of sexual health education on the promotion of sexual function, especially in pregnant women with diabetes, Mansouri et al. (2020) examined the effect of sexual health education based on the health belief model and Pender’s health promotion model on the sexual performance of women with type 2 diabetes and found that the use of both training models can significantly improve all aspects of sexual function in diabetic women, and there was no significant difference between the two groups. However, the health belief model was significantly more efficient than Pender’s health promotion model in terms of psychological stimulation (27). Nezamnia et al. (2020) examined the effectiveness of cognitive-behavioral therapy in sexual function in pregnant women, and showed that this treatment was effective in improving all domains of female sexual function two and four weeks after the psychological intervention (28). These findings are consistent with the results of the present study in terms of the impact of intervention techniques on all aspects of sexual function, while the training and intervention techniques in the two studies were slightly different.
Marvi et al. (2019) examined the effect of sexual health model training on the sexual function of women with infertility and showed that sexual health training had a significant effect on improving sexual function, including desire, lubrication, arousal, and orgasm in infertile women with serious psychological problems (29). Esposito et al. (2004) examined the effect of lifestyle change on sexual function in obese men with erectile dysfunction and found that using a healthy lifestyle improves sexual function in these men (30). Following the findings of the present study, these studies showed that psychological training of any kind can have positive and significant changes in improving the quality of sexual life of patients (31). Given that healthy lifestyle education can promote various aspects of mental health and sexual function in women of reproductive ages, sexual function can be improved with effective and standard training interventions (32).
The results of the present study showed that in addition to the group receiving sexual health promotion training, the participants in the control group who received no training intervention reported significant changes in their sexual function, especially in the domains of arousal, lubrication, orgasm, and pain. Perhaps factors such as receiving routine training and care during pregnancy from comprehensive health centers, possible visits to the doctor for GDM, and pregnancy progression changed the sexual function of the women in the control group.
Hashem et al. (2020) studied the effect of sexual health promotion training programs on the sexual function of pregnant women and found that implementing such training programs can significantly improve the sexual function of pregnant women. It was also shown that the sexual function of pregnant women was significantly different in each trimester (33). These findings provided empirical support for the results of the present study indicating the significant improvement in sexual function of pregnant women with increasing gestational age. However, Davari-Tanha et al. (2020) examined female sexual dysfunction in each trimester and showed that the difference in sexual function in the second trimester was less than in the first and third trimesters (34). Safaralinezhad et al. also showed that increasing gestational age from the first trimester to the third trimester caused a gradual increase in sexual dysfunction in each trimester compared to the previous trimester (35). The results of these studies were not in line with the present study. Perhaps the reason for this discrepancy was that the intervention in the present study began during the first trimester of pregnancy.
According to the findings of previous studies and the present study, it can be concluded that education in women at risk of decreased sexual function, especially women with GDM, can enhance all domains of sexual function, and ultimately improve the quality of life in these women. However, despite the prohibition of discussing sexual issues in some cultures, including the Iranian community, various studies have shown that sex education is effective and sexual issues are influenced by any direct or indirect education.
Some of the limitations of this study included difficulty in talking about sexual issues and problems due to cultural norms of Iranian society, the impossibility of men’s participation in the training program, impossibility of forming larger training groups due to the COVID-19 outbreak, and not examining the retention effects of the training program in the postpartum period.
5.1. Conclusion
The results of present study showed that the sexual health promotion training program promoted overall sexual function and its domains in pregnant women with GDM. Given the effectiveness of this training intervention, it is suggested that the sexual health promotion program developed in this study be integrated into pregnancy care programs for women with GDM to increase the health and well-being of this group of vulnerable women.