1. Background
Cancer is a chronic disease caused by cell deformation through genetic mutations in DNA. The altered cell escapes regulatory mechanisms and eventually invades surrounding tissues and reaches lymphatic or blood vessels (1). Cancer is considered to be the major health problem of the century and its increasing growth in the last two decades and its negative effects on the physical, psychological, social, and economic aspects of the patient’s life are the concern of health experts more than ever before (2). Breast cancer is the most common cancer among women in the world and Iran. According to the latest World Health Organization (WHO) statistics, the incidence of breast cancer in the world was 2,088,849 cases in 2018, accounting for 11.6% of all cancers, and this rate in Iran was 13,776 cases in 2018, comprising 12.5% of all cancers across the country (3).
With technological advancements, huge steps have been taken to treat breast cancer using treatments such as surgery, chemotherapy, radiotherapy, and hormone therapy. These techniques have complications despite increasing patients’ longevity (4). One of the most principal and basic treatments for breast cancer is surgery, which is performed in several ways depending on the disease progression. In surgery, the lymph nodes are removed depending on the extent of cancer involvement (1). The most debilitating and common complications after breast cancer surgery are lymphedema, pain, and decreased limb function (5). Studies have shown that over 94% of breast cancer patients experience varying degrees of lymphedema of the arm (6). Lymphedema is a chronic, progressive, and deforming complication (7) associated with a feeling of heaviness and stiffness, limited range of motion (ROM), limb pain, extreme fatigue, decreased daily function, and fine and gross motor disabilities that lead to the inability to take care of oneself and disruption of one's social relationships (6). Lymphedema can lead to limb dysfunction and pain, and due to the chronic nature of this complication, the prevention and maintenance of limb function are vital. Thus, self-care of the affected limb and continued care until the end of life after surgery play an important role in reducing the incidence of lymphedema, and consequently, preserving the affected limb. If lymphedema is not treated, edema increases (1), and fibrotic tissue develops in the affected limb, which in turn leads to neurological disorders, such as pain and loss of sensation, heaviness and disability, limb infection, or bacterial and fungal infections of the skin (8), and paves the way for lymphangiosarcoma (9, 10).
Feiten et al. reported the rate of shoulder and arm dysfunction in patients with a history of breast cancer surgery to be 46%, which directly affects a person's personal and social activities and highlights the need for self-care (11). In a study conducted by Hopkins et al, the limited range of motion of the arm in patients with a history of breast cancer surgery was reported to be 51%. Furthermore, in the same study, the rate of arm and shoulder pain in these patients was assessed and reported to be 63% (12). Ridner et al. reported a 20% decrease in physical activity following lymphedema and a 21% inability to raise the affected arm, leading to the patient’s gradual dependence on family and caregivers. They suggested that early self-care can be effective in maintaining individual independence (13).
The lack of definitive treatment for lymphedema and the patient’s tendency to immobilize the affected limb to create a greater sense of comfort lead to the progression of the complication and limb pain and dysfunction; thus, there is a need for more self-care training. The goal of self-care is to prevent edema, reduce swelling, restore limb function, and diminish limb pain and discomfort, hence, improving patients' quality of life (14). According to the WHO, self-care is the personal, family, and social ability to promote health, prevent disease, maintain health, and combat disease, with or without the support of healthcare providers (15).
Following the breast cancer-related lymphedema guidelines, various conservative therapies for arm lymphedema secondary to breast cancer treatment have been proposed, including lymphatic drainage self-massage, compression bandaging / garments, limb exercise, and careful skincare, each to be performed at different times (16). According to the International Society of Lymphology (ISL), the treatment of lymphedema includes conservative and surgical therapies. The best and most effective treatment for lymphedema is a mixed decongestant, which is a conservative treatment and includes manual lymphatic drainage massage, skincare, and hygiene, compression garments to reduce edema and limb pain, and recommended exercises to improve limb function (17, 18). Self-care in lymphedema is often referred to as “risk reduction techniques” and “self-management of lymphedema symptoms” that include behaviors and activities performed by the individual with or without the help of others (5). Various complications of chronic diseases can be controlled through self-care behaviors. These behaviors emphasize evaluating and controlling disease symptoms, accepting the treatment regimen, maintaining a healthy lifestyle, and controlling the impact of the disease on daily functioning, emotions, and social relationships (19). Providing cancer patients with information about the diagnosis, treatment, and ways to reduce complications can help patients better engage in the decision-making process, ultimately leading to effective treatment and reduction of complications (20). Considering the significant effects of lymphedema on the physical, psychological, and functional aspects of patients’ lives, further studies are needed to address the techniques used to reduce lymphedema symptoms.
2. Objectives
The majority of studies have only focused on manual lymphatic drainage (MLD) massage. However, the present study aimed to explore the effectiveness of self-care training in upper limb function and pain after breast cancer surgery. Thus, it is different from other studies conducted in Iran. Afterward, self-care training with specific content was implemented in this study. The insights provided by this study can be used to control some of the serious problems of breast cancer patients in the future and improve their quality of life.
3. Methods
This quasi-experimental study was performed with a pretest-posttest design. The sample size was determined as 14 persons per group using the following formula with a 95% confidence interval and 95% test power. However, to increase the rigor of the study, the number of participants in each group was determined to be 30 persons (60 persons in total).
The participants in this study were 60 breast cancer patients admitted to Khatam Al-Anbia and Ali Ibn Abi Taleb (AS) hospitals. The patients were selected using convenience sampling. The inclusion criteria were patients aged 20 to 65 years, patients with 30 ≥ BMI, patients with a history of radical / modified / total / mastectomy and lumpectomy, and removal of at least 2 lymph nodes during surgery, patients with no lymphedema or pain at the beginning of the study, unilateral breast involvement, undergoing the first chemotherapy session, no pregnancy and lactation, no diabetes / musculoskeletal problems / central-peripheral nervous system disorders, and voluntary participation in the study. The exclusion criteria were patient death, developing other cancers during the study, metastasis to other tissues during the study, not attending more than one training session, and not doing exercises. The patients were selected using convenience sampling and divided into two groups (control and intervention) via random allocation by selecting color cards. To this end, envelopes containing color cards bearing the group names were prepared, and each card was assigned to a patient who met the inclusion criteria to be assigned to either the control or intervention group.
The data in this study were collected using three instruments that were administered to the members of the two groups before, one month, and three months after the intervention.
3.1. The Demographic Information Form
It collected information about patients’ age, marital status, number of children, education, occupation, and type of surgery. The form was completed by the patients only at the beginning of the study.
3.2. Disabilities of the Arm, Shoulder, and Hand Questionnaire
The questionnaire contains 30 items measuring the symptoms and function of the upper limb involved in orthopedic and neurological disorders. Each item is scored on a five-point scale ranging from 0 (no disability) to 5 (most severe disability). The total score is calculated as the sum of the scores of the items and varies from 0 to 150. A higher score indicates more involvement (150 means severe disability), and a lower score is a sign of low involvement of the upper limb (a score of 30 means no disability). The Cronbach's alpha coefficient for the Persian version of this questionnaire was calculated to be 0.96 (21). The reliability of the questionnaire in the present study was estimated as 0.85.
3.3. McGill Pain Questionnaire
The questionnaire has 20 items that examine people's perception of pain in terms of sensory perception, emotional perception, evaluation perception, as well as various pains. The items are scored based on the table of pain dimensions. The total score ranges from 0 to 82. This questionnaire was reviewed for use in Iran, and its validity and reliability were confirmed. For example, Khosravi et al. reported the total Cronbach's alpha of this questionnaire as 0.85, and Cronbach's alpha of all four components above 0.80 (22). In the present study, Cronbach's alpha for this questionnaire was 0.85.
After random allocation, the participants in the control group did not receive any special care except for the routine care and only received a training booklet and CD at the end of the study. However, the patients in the intervention group, in addition to the routine care, received the necessary self-care training based on common and potential problems faced by breast cancer patients in five 45-minute sessions individually in the oncology ward. The training programs focused on questions and answers, face-to-face training, and practical training with specific content and the use of training booklets and CDs prepared based on the latest scientific resources (Table 1) (23). In the last two sessions, lymphedema preventive manual massage and exercises were instructed and performed practically based on the content of the exercise table in the booklet. To ensure the patient performed the exercises, a checklist was given to the patient to be completed weekly and submitted to the researcher. The patients’ arm circumference was measured using a meter, and their upper limb function and pain were measured with Disabilities of the Arm, Shoulder, and Hand (DASH) and McGill pain questionnaires one and three months after the self-care training intervention. Similar evaluations were also performed for the patients in the control group.
| Sessions | Description | Duration |
|---|---|---|
| 1 | 1. Welcoming the group members and introducing them; 2. Describing the training program, the number of sessions, and their duration; 3. Giving some information about breast cancer, surgical techniques, and related complications; 4. introducing the lymphatic system and its function; 5. Defining lymphedema and signs and symptoms | 45 minutes |
| 2 | 1. Introducing the causes of lymphedema; 2. Discussing lymphedema risk factors; 3. discussing the effect of lymphedema on a person's life; 4. Reviewing lymphedema changes over time | 45 minutes |
| 3 | 1. Discussing risk factors for lymphedema; 2. Introducing different types of lymphedema treatments; 3. Introducing compression methods of lymphedema treatment; 4. Arm skincare training | 45 minutes |
| 4 | 1. Introducing manual lymphatic drainage (MLD); 2. Performing manual lymphatic drainage (MLD) massage | 45 minutes |
| 5 | 1. Introducing lymphedema exercises; 2. Performing Introducing lymphedema exercises | 45 minutes |
The collected data were coded and analyzed by SPSS version 22. First, the upper limb function and pain scores for the participants in the two groups were determined using descriptive statistics, including frequency, percentage, mean, standard deviation, minimum, and maximum. Moreover, repeated measures analysis of variance (ANOVA) and the Bonferroni test were used to compare the mean scores of upper limb function and pain in the two groups one month and three months after the intervention. Chi-square test was used to compare the frequency of qualitative variables in the two groups. The significance level in this study was considered less than 0.05 (P < 0.05).
4. Results
The mean and standard deviation of the patients’ age in the intervention and control groups were 4.2 ± 6.25 and 40.6 ± 7.65 years, respectively. Moreover, 26.7% of the patients in the two groups had high school and lower education, and 40% of the patients in the two groups were housewives. It was also shown that 41.7% of patients in the intervention group and 40% of patients in the control group were married. There was no statistically significant difference between the two groups in terms of demographic variables, including age, education, and occupation (P > 0.05). The most common type of surgery was modified radical mastectomy that had been performed for 48.3% of the patients in the two groups (Table 2).
| Variables | Intervention Group | Control Group | P-Value |
|---|---|---|---|
| Marital status | 0.73 b | ||
| Married | 25 (83.3) | 24 (80) | |
| Single | 5 (16.7) | 6 (20) | |
| Education | 1 b | ||
| High school and lower education | 16 (53.3) | 16 (53.3) | |
| Diploma and higher education | 14 (46.7) | 14 (46.7) | |
| Occupation | 1 b | ||
| Employed | 6 (20) | 6 (20) | |
| Housewife | 24 (80) | 24 (80) | |
| Age | 0.19 c | ||
| Frequency | 30 | 30 | |
| Min - max | 32 -55 | 32 -55 | |
| Mean ± SD | 41.2 ± 6.25 | 40.6 ± 7.65 |
a Values are expressed as No. (%) unless otherwise indicated.
b Fisher’s exact test.
c Independent samples t-test.
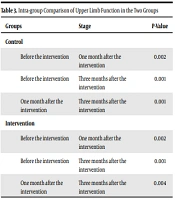
The results of repeated measures ANOVA (Table 3) indicated that the mean score of upper limb function in the control group was significantly lower one month and three months after the intervention than the mean score before the intervention (P < 0.002 vs. P < 0.001), indicating that patients' upper limb function was significantly impaired. However, the mean score of upper limb function in the intervention group increased significantly one month and three months after the intervention compared to their mean scores before the intervention (P < 0.002 vs. P < 0.001), indicating that the upper limb function enhanced significantly in the patients in the intervention group compared to the patients in the control group.
| Groups | Stage | P-Value |
|---|---|---|
| Control | ||
| Before the intervention | One month after the intervention | 0.002 |
| Before the intervention | Three months after the intervention | 0.001 |
| One month after the intervention | Three months after the intervention | 0.001 |
| Intervention | ||
| Before the intervention | One month after the intervention | 0.002 |
| Before the intervention | Three months after the intervention | 0.001 |
| One month after the intervention | Three months after the intervention | 0.004 |
As shown in Table 4, time and group effects had a significant interaction, demonstrating that the pattern of function score changes was different between the two groups before the intervention and one and three months after the intervention (P = 0.001). Furthermore, the data presented in Table 5 indicate that the mean scores of upper limb function one and three months after the study were higher in the intervention than the control group, and this difference was statistically significant only three months after the intervention (P < 0.001). In other words, the quality of the upper limb function improved in the intervention group.
| Source of Changes | Sum of Squares | df | Mean Squares | F | P-Value | Effect Size | Power |
|---|---|---|---|---|---|---|---|
| Time | 120 | 1 | 120 | 1.03 | 0.31 | 0.01 | 0.17 |
| Group | 3489.08 | 1 | 5489.08 | 6.78 | 0.01 | 0.10 | 0.72 |
| Time-group interaction | 6660.30 | 1 | 6660.30 | 57.47 | 0.001 | 0.49 | 1 |
| Error | 46896.44 | 1 | 808.55 |
| Stage | DASH Score ± SD | Bonferroni Test (Intergroup Comparison) | |
|---|---|---|---|
| Intervention Group | Control Group | ||
| Before the intervention | 55.8 ± 21.2 | 53 ± 18.8 | P = 0.56 |
| One month after the intervention | 49.9 ± 17.5 | 58.9 ± 18.9 | P = 0.06 |
| Three months after the intervention | 42.9 ± 8.5 | 69.9 ± 19 | P = 0.001 |
a Values are expressed as DASH score ± SD.
The results of repeated measures ANOVA) (Table 6) indicate that the mean score of pain in the control group was significantly higher one month and three months after the intervention than the mean score before the intervention, implying that the pain score reported by the patients was significantly higher after the intervention compared to the pre-intervention period (P < 0.001). However, the mean score of pain in the intervention group decreased significantly one and three months after the intervention compared to their mean scores before the intervention (P < 0.001 vs. P < 0.004). As can be seen in Table 7, time and group effects had a significant interaction, suggesting that the pattern of pain score variation was different between the two groups before the intervention and one month and three months after the intervention (P = 0.001). The data in Table 8 show that the mean pain scores before the intervention and one and three months after the intervention were 10.4, 35.7, and 6.26 for the intervention group and 10.8, 41.7, and 21.1 in the control group, respectively. Thus, the pain mean score in the intervention group was significantly lower than that of the control group three months after the intervention (P = 0.001).
| Groups | Stage | P-Value |
|---|---|---|
| Control | ||
| Before the intervention | One month after the intervention | 0.001 |
| Before the intervention | Three months after the intervention | 0.001 |
| One month after the intervention | Three months after the intervention | 0.001 |
| Intervention | ||
| Before the intervention | One month after the intervention | 0.001 |
| Before the intervention | Three months after the intervention | 0.004 |
| One month after the intervention | Three months after the intervention | 0.001 |
| Source Of Changes | Sum of Squares | df | Mean Squares | F | P-Value | Effect Size | Power |
|---|---|---|---|---|---|---|---|
| Time | 282.13 | 1 | 282.13 | 12.40 | 0.01 | 0.17 | 0.93 |
| Group | 2247.20 | 1 | 2247.20 | 21 | 0.001 | 0.26 | 0.99 |
| Time-group interaction | 1555.20 | 1 | 1555.20 | 68.35 | 0.001 | 0.54 | 1 |
| Error | 1319.66 | 58 | 22.75 |
| Stage | Mcgill Pain Score ± SD | Bonferroni Test (Intergroup Comparison) | |
|---|---|---|---|
| Intervention Group | Control Group | ||
| Before the intervention | 10 ± 0.90 | 10.83 ± 0.90 | P = 0.73 |
| One month after the intervention | 35.7 ± 2.39 | 41.7 ± 2.39 | P = 0.85 |
| Three months after the intervention | 6.26 ± 1.12 | 21.1 ± 1.12 | P = 0.001 |
5. Discussion
This study evaluated the effect of self-care training on upper limb function and pain after surgery in patients with breast cancer. The findings indicated that with self-care training, the upper limb function of the mastectomy patients improved significantly three months after the intervention compared to patients in the control group. These results were in line with the finding of a study by Khosh Nazar et al. on the effect of home-based rehabilitation on lymphedema volume and daily hand activity in women after mastectomy. However, the methodology taken in the above study was different from that of the present study. Khosh Nazar et al. used a researcher-made questionnaire to assess the function of the hands and the whole upper limb. However, in the present study, the DASH questionnaire was employed to measure the upper limb function in four points. The authors found that the rehabilitation program improved hand activity in mastectomy patients (24). In line with the findings of the present study, Sezgin Ozcan et al. used complex decongestive therapy to improve upper limb function in patients with breast cancer-related lymphedema using a methodology that was similar to the procedure taken in the present study. The results indicated that complex decongestive therapy improved upper limb function (25). Moreover, Corrado et al. confirmed the effectiveness of a home-based exercise program on upper limb function and the quality of life in breast cancer survivors, as was supported by the data in the present study. The difference between this study and the present study could be attributed to the assessment tool. The authors used the Constant-Murley score (CMS) to assess shoulder function. The results showed that the exercise program increased the range of motion of the upper limbs and improved shoulder and elbow function (26). Ghorbani and Sokhangouei compared the effects of three exercise methods (ie, Pilates, yoga, and physical activity) on the range of motion and upper extremity edema and body image in women with breast cancer after mastectomy. It was found that yoga and Pilates were effective in increasing the range of motion of the upper limbs. The authors concluded that in addition to self-care training, the use of other methods can be effective in improving upper limb function (27). Mokhtari Hesari et al. examined the effect of exercise and complete decongestive therapy on edema volume and shoulder range of motion in patients with breast cancer-related lymphedema and showed that aerobic exercise, resistance exercise, and mixed exercise had no significant impact on lymphedema and upper limb function even in interventions for more than or less than 12 weeks (28). This finding was inconsistent with the results of the present study. The main focus of the present study was on the self-care program, part of which included exercises that increased the range of motion but and did not consider a specific type of exercise. Perhaps one of the reasons affecting the results of the present study was that the patients were selected based on the type of surgery and the number of nodes removed. Thus, the present study was different from the majority of studies that have addressed patients with breast cancer who underwent breast cancer because the number of nodes removed is one of the factors that affect the severity of lymphedema. Different studies have also shown that resistance training as well as mixed therapy exercises consisting of physiotherapy or movement therapy for breast cancer patients with lymphedema and upper extremity dysfunction are safe and can prevent secondary lymphedema (29-31). The self-care training program conducted in the present study focused on total factors affecting limb function and edema. It seems that self-care training programs can be used as a strategy to improve upper limb function.
Pain intensity was lower for the participants in the intervention group than those in the control group one month after the intervention, but it was not significant. However, three months after the intervention, pain intensity was reported to be significantly lower for the patients in the intervention group. Accordingly, it can be argued that with the application of massage therapy, the lymph first goes to the upper parts but is not drained, and this increases the accumulation of pain, but gradually the lymph begins to drain in the areas, and as a result, the pain is reduced. Then, the lymph reaches the proximal area of the arm by massaging the forearm, and there is a relative obstruction in this area; thus, the lymph comes out of this area slowly after the massage. Lymph accumulation in this area causes pain. Studies have shown that lymph drainage is delayed at the beginning of massage and physiotherapy treatment, but then it is drained faster. It has also been shown that the massage of the hands, neck, and armpits sometime later helps to drain the lymph by creating sub-canals (32). In line with the present study, Sheikhi Mobarakeh examined the impact of combined decongestive therapy on the reduction of pain and heaviness in patients with breast cancer-related lymphedema and showed that combined decongestive therapy, including lymphatic drainage massage, bandaging, use of compression sleeves, exercise, and self-care can significantly reduce the complications of lymphedema, and ultimately, the pain of patients, (33). However, this study was different from the present study in terms of methodology and the instruments used. The present study employed the McGill Pain Questionnaire (MPQ) that specifically measures different dimensions of pain. This was one of the strengths of the present study compared to other studies. However, given that McGill Pain Questionnaire (MPQ) is a multidimensional instrument, this study provided a general review of this instrument, and a thorough analysis of its dimensions requires more comprehensive studies.
5.1. Conclusions
The results of the present study showed that providing self-care training for women with breast cancer who have undergone mastectomy is effective in maintaining function and reducing pain in the affected limb. Thus, self-care training can be used as a simple and low-cost method to improve limb function and prevent pain. The cost of self-care training is recommended to be studied. One of the strengths of the present study was the careful selection of patients by taking into account the factors affecting lymphedema, which was not the case in other studies. Moreover, the DASH questionnaire was employed in this study to measure the upper limb function in four points.
5.2. Limitations
In this study, due to time constraints, the follow-up phase was performed up to three months after the intervention, while complications such as lymphedema, upper limb dysfunction, and pain can appear long after cancer treatment and are even intensified over time. Thus, similar studies need to be performed over longer periods.