1. Background
Pregnancy-induced hypertension (PIH) is one of the most common problems during pregnancy that causes mortality and morbidity to the mother, fetus, and neonate (1). Hypertensive disorders during pregnancy include chronic hypertension, preeclampsia-eclampsia, preeclampsia superimposed on chronic hypertension, and gestational hypertension (2). Preeclampsia is characterized by PIH plus proteinuria or edema occurring after the 20th week of gestation. Hypertension without other symptoms of preeclampsia during pregnancy is called transient hypertension, while it is called chronic hypertension if occurring before the pregnancy or blood pressure levels of ≥ 140/190 mm Hg before the 20th week of gestation (2, 3).
Hypertensive disorders of pregnancy complicate about 5 - 10% of pregnancies (3). The incidence of hypertensive disorders during pregnancy is increasing and is becoming the leading cause of maternal mortality and morbidity (4, 5). It is associated with severe complications for mothers, such as eclampsia, pulmonary edema and hemolysis, elevated liver enzymes, and HELLP syndrome in 5 - 10% of pregnancies (5-11). The capillary damage caused by PIH affects the feto-placental unit. Therefore, prolonged PIH can lead to reduced blood flow, uteroplacental insufficiency, placental abruption, fetal distress, intrauterine growth restriction (IUGR), low birth weight, low Apgar score, preterm labor, and stillbirth (11-14).
A range of hematological changes may be observed in infants of PIH mothers. Reports indicate an increase in the nucleated red blood cell (RBC) counts in infants of mothers with secondary gestational hypertension and decreased fetoplacental blood flow. In addition, a higher risk of polycythemia has been reported in these infants (14, 15).
Neutropenia and neonatal infections, especially in premature infants, are other hematological changes observed in the neonates of mothers with preeclampsia (16). Studies are limited to changes in blood parameters of infants of hypertensive mothers.
2. Objectives
This study compared the hematological parameters between neonates of mothers with preeclampsia and those of controls. Based on the admission of some of these infants in the neonatal ward and conducting various tests, the results can help interpret the tests more accurately.
3. Methods
The study population included 30 neonates born to mothers with PIH and 30 neonates of normotensive mothers admitted to the Ali ebne Abitaleb Hospital in Zahedan from January 2016 to May 2017.
The exclusion criteria were as follows: newborns with congenital or chromosomal anomalies, asphyxia, congenital metabolic diseases, perinatal infections, hematologic and endocrine diseases; neonates with intrauterine age < 24 weeks; stillborn infants, infants born to mothers with chronic illnesses who had taken medications other than antihypertensive drugs; and those who had encountered a premature rupture of membranes.
Demographic data of the mothers and the newborns, such as age, gestational age, and neonate gender, were recorded. Immediately after childbirth and after the umbilical cord cutting, 2 ml of cord blood was taken for complete blood count (CBC) testing and ferritin level measurements. The specimens were stored under suitable conditions. Also, CBC indices including white blood cell (WBC), RBC, hemoglobin (HB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), platelet, mean platelet volume (MPV) and platelet distribution width (PDW) were measured using the Sysmex KX-21N cell counter device (Japan). Plasma ferritin was measured using the ELISA method. Written informed consent was obtained from the patients. The ethics committee of Zahedan University of Medical Sciences approved the study (IR.ZAUMS.REC.1394.270). Descriptive and analytical statistics were performed using the SPSS software version 21. Independent t-test and Mann-Whitney test were used to compare data between the two groups. The P value < 0.05 was considered statistically significant.
4. Results
This study compared the hematological parameters of 30 neonates born to mothers with preeclampsia and 30 infants of normotensive mothers as the control group.
The mean age of mothers in the case and control groups was 29.36 ± 5.65 and 29.03 ± 6.56 years, respectively (P > 0.05). The mean gestational age of infants in the case and control groups was 37.26 ± 2.28 and 38.36 ± 1.15 weeks, respectively (P > 0.05). In the case group, 13 (43.3%) neonates were female, and 17 (56.7%) neonates were male, while there were 17 (56.7%) female neonates and 13 (43.3%) male neonates in the control group. The mean weight of neonates was 2968 ± 445 grams and 3203 ± 329 grams in the case and control groups, respectively (P = 0.02).
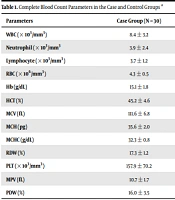
Table 1 compares the umbilical cord white blood cell, red blood cell, and platelet indices in the two groups.
| Parameters | Case Group (N = 30) | Control Group (N = 30) | P Value |
|---|---|---|---|
| WBC (× 103/mm3) | 8.4 ± 3.2 | 11.3 ± 3.4 | 0.002 |
| Neutrophil (× 103/mm3 | 3.9 ± 2.4 | 5.7 ± 2.9 | 0.01 |
| Lymphocyte (× 103/mm3) | 3.7 ± 1.2 | 4.6 ± 2.3 | 0.06 |
| RBC (× 106/mm3) | 4.3 ± 0.5 | 4.3 ± 0.5 | 0.62 |
| Hb (g/dL) | 15.1 ± 1.8 | 14.2 ± 1.9 | 0.07 |
| HCT (%) | 45.2 ± 4.6 | 44.5 ± 6.1 | 0.62 |
| MCV (fL) | 111.6 ± 6.8 | 104 ± 10.0 | 0.001 |
| MCH (pg) | 35.6 ± 2.0 | 33.6 ± 3.6 | 0.01 |
| MCHC (g/dL) | 32.3 ± 0.8 | 32.1 ± 1.1 | 0.56 |
| RDW (%) | 17.3 ± 1.2 | 12.1 ± 1.1 | < 0.0001 |
| PLT (× 103/mm3) | 157.9 ± 70.2 | 262.4 ± 62.8 | < 0.0001 |
| MPV (fL) | 10.7 ± 1.7 | 9.7 ± 0.8 | 0.005 |
| PDW (%) | 16.0 ± 3.5 | 11.7 ± 0.8 | < 0.0001 |
Complete Blood Count Parameters in the Case and Control Groups a
The mean MCV, MCH, RDW, PDW, and MPV was significantly higher in neonates of mothers with preeclampsia than in those of the control group (P < 0.05).
The mean ferritin was 162 ± 126 ng/mL and 254 ± 101 ng/mL in neonates of the case and control groups, respectively (P = 0.003).
In addition, the mean WBC, platelet, and ferritin was significantly lower in neonates of the case group than in those of the control group (P < 0.05). The mean neutrophil percentage, lymphocyte percentage, RBC count, HCT, hemoglobin, and MCHC was not significantly different in the two groups (P > 0.05).
5. Discussion
Preeclampsia changes the intrauterine environment of the fetus. The fetus has to adapt itself to the unfavorable environment. The adverse effects of preeclampsia on the fetus and infant include increased neonatal mortality and morbidity, intrauterine growth restriction, premature birth, hematological changes, necrotizing enterocolitis, and bronchopulmonary dysplasia (17). The cytotoxic environment present in preeclampsia affects the development of fetal cell lineages. Neutropenia is observed in 50% of neonates and is correlated with mortality (18).
Currently, there are no significant paraclinical findings for interpreting hematological findings in infants born to mothers with PIH. This research studied 60 neonates to compare the hematological parameters in neonates of hypertensive and normotensive mothers. Each group included 30 neonates.
The comparison of hematological parameters in the two groups showed that the average dispersion range of MCV, MCH, RDW, PDW, and MPV was significantly higher in infants of the case group than in those of the control group. In addition, the mean WBC, platelet count, and ferritin was significantly lower in infants of the case group than in those of the control group.
The mean of other hematologic parameters including neutrophil percentage, RBC count, HCT, hemoglobin, and MCHC was higher in newborns of the case group compared to those of the control group; however, the differences were not significant.
Thrombocytopenia has been confirmed to occur in neonates of hypertensive mothers in several studies (19).
The mechanism of thrombocytopenia among neonates born to mothers with preeclampsia is currently unknown; however, previous studies have shown that in mothers with preeclampsia, fetal hypoxia imposes some adverse effects on platelet production by bone marrow megakaryocytes (10, 17).
There is also evidence of direct and indirect effects of the placenta on thrombocytopenia. For example, in hypertensive mothers, vasospasm and segmental vasodilation of the placenta bind platelet to damaged endothelium and destroy it (20). Therefore, PIH can lead to thrombocytopenia in neonates of these mothers with several mechanisms. This study showed a reduction in the WBC count in hypertensive mothers. This finding confirms the results of studies by Bloat et al. (18) and Prakash et al. (21).
However, Sivakumar et al. showed no significant difference between neonates of hypertensive and non-hypertensive mothers in terms of the WBC count and neutrophil count, which is not consistent with the result of the present study (22). Inhibition of the production of bone marrow myeloid cells due to uteroplacental insufficiency caused by PIH is among the possible mechanisms in this regard. On the other hand, PIH is a multi-systemic disorder that may affect the inflammatory system. An increased number of neutrophils in neonates of hypertensive mothers may be a clinical manifestation of underlying pathogenesis. However, this difference was not significant in this study. The low sample size in this study and the probability of occurrence of this condition in infants suffering from these complications can partly justify this issue.
Another study finding showed an increase in various RBC indices in newborns of hypertensive mothers, and the differences were significant in most cases. Kurlat and Sola showed that the risk of incidence of polycythemia was 12.6 times greater in infants of hypertensive mothers than in those of the general population (14). A study by Bolat et al. also indicated an increase in the RBC count and hemoglobin levels in infants of hypertensive mothers compared to those of the control group (18). The findings of studies by Sivakumar et al. (22), Prakash et al. (21), and Catarino et al. (23) indicated an increase in the MCV and immature RBC count in newborns of hypertensive mothers compared to those of the control group. They showed no significant difference in hemoglobin, hematocrit, MCH, and MCHC.
A study by Catarino et al. showed no significant difference between the neonates of mothers with preeclampsia and those of healthy mothers regarding the RBC count and hemoglobin and hematocrit concentrations. However, the MCV, MCH, MCHC, and reticulocyte counts were higher in neonates of hypertensive mothers. The results of this study are consistent with the findings of our study (23).
Another study showed significant positive correlations between preeclamptic mothers and their newborns in Hb, PCV, MCV, MCHC, RBC, and retics (24).
It seems that uteroplacental insufficiency and prolonged exposure to hypoxia increase erythropoietin levels, subsequently increasing erythropoiesis. Previous studies have mainly reported an increase in premature RBC in peripheral blood smear (5, 17, 23, 25) or reticulocyte (19, 21, 25).
In this study, a peripheral blood smear was not examined. However, the increased MCV in infants of the case group may signify the presence of premature RBC in peripheral blood, mainly because the differences between MCH and MCHC levels have not been reported as significant in most studies. Given the low number of neonates in the present study, further studies are needed to confirm obtained findings.
Meher et al. documented only a change in neutrophil count in newborns of hypertensive mothers in comparison to the newborn of normotensive women (26). Another study showed a decrease in the platelet count and an increase in the platelet indices, like mean platelet volume and platelet distribution width, in preeclampsia women compared to normotensive women (27).
The study of Bolat et al. was the only study to examine the serum ferritin levels in newborns of hypertensive mothers and found that ferritin levels were significantly lower in newborns of hypertensive mothers, which is consistent with the findings of our study (18).
In conclusion, birth weight and the WBC, neutrophil, and platelet counts were lower in newborns of the case group because of uteroplacental insufficiency compared to those of the control group. Therefore, hematologic follow-up should be performed on these neonates. Complete blood counts can be used for early diagnosis of hematologic abnormalities in neonates of mothers with preeclampsia.