1. Background
Infancy is a very important and critical period. Therefore, providing, maintaining, and promoting the level of neonatal health as an important indicator of development has a special place in health services. One of the problems of infancy is prematurity (1). According to the World Health Organization, live neonates born before the 37th week of pregnancy from the first day of the last menstrual period are considered premature (2, 3). Prematurity is the most important cause of death in neonates without congenital abnormalities. Preterm birth accounts for about 8% - 10% of all births worldwide. Today, about 15 million premature births (about 1 in 10 neonates) occur worldwide annually, with a prevalence rate of 12% and 40% in developed and developing countries, respectively (4, 5). In the 21st century, this problem accounts for more than two-thirds of neonatal deaths in developed countries. Four million neonates are born in the United States every year, with an average of 12.5% being premature. In Bulgaria, statistics showed that 10% - 12% of all pregnancies end in premature birth (6, 7). Iran is also one of the regions with a high prevalence of premature birth, where the rate of premature birth is 5.14% - 6.4%, and according to the statistics of the Iranian Ministry of Health and Medical Education, its prevalence rate is estimated as 8% (8).
Prematurity causes a high percentage of death, as well as short- and long-term complications in neonates (9). Premature neonates are at a high risk of mental disorders (e.g., autism spectrum disorder, attention deficit hyperactivity disorder, anxiety, and depression), motor and sensory disorders (e.g., problems related to vestibular balance, pain processing, deafness, and cerebral palsy), delay in development (language, cognitive, sensory and motor development), and academic performance which is weaker compared to term neonates. The exact cause of these problems is unclear (10, 11). These conditions not only create an emotional burden for families but also impose an economic burden on society (12).
Today, progress in medical science and nursing care has led to an increase in the survival of premature neonates who need hospitalization in the neonatal intensive care unit (NICU) (7, 9, 13) The NICU bears little resemblance to the mother's womb and exposes the infants to countless sensory stimuli, including high levels of noise, intense light, and frequent medical procedures. This unusual amount of sensory stimulation experienced by premature neonates can adversely affect their developing brains and lead to long-term neurodevelopmental problems (14, 15). Exposure of premature neonates to excessive noise can activate the autonomic stress and hypothalamic-pituitary-adrenal systems, leading to important physiological changes, including increased blood pressure and heart rate, apnea, hypoxia, bradycardia, changes in oxygen saturation, and augmented secondary oxygen consumption due to increased heart rate and breathing (16-18).
Physiological functions and behavioral characteristics that are necessary to adapt to life in the extra-uterine environment must be completed during the transfer process. However, this is impossible in premature neonates. The interaction between the neonate and the environment is influenced by physiological differences and care measures. Physiological parameters of premature neonates indicate that their health status and changes in them are the most important and first signs of changes in health (19). The combination of pain, stress, separation from parents, environmental stimuli, and multiple caregivers may have a negative effect on the health of neonates, manifested as changes in physiological states, such as heart rate, breathing, temperature, skin color, and oxygen saturation levels, wide fluctuations in blood pressure, and increased restlessness in the newborn (20, 21). Therefore, the NICU should provide a situation for neonates in which their development is possible with minimal damage (19).
Considering the role of environmental factors in the sleep disorder of patients hospitalized in the NICU, it is necessary to use a suitable protocol to improve the sleep of these patients. In the quiet time protocol, nurses use non-invasive, uncomplicated, and cost-free approaches, such as a program designed to calm the environment both physically and psychologically, to improve the quality of the sleep of patients (22, 23). The "quiet time protocol" or "quiet hour protocol" intervention includes a specific period consisting of 8-hour shifts during which light and sound are controllably reduced, and irritations at the patient's bedside are minimized (24, 25). A protocol is designed to improve the environment by focusing on minimizing sound by including private rooms, educating staff about the negative effects of sound, keeping staff quiet, minimizing patient handling, turning down the alarm sounds, or setting them to vibration mode, using visual warning systems, responding immediately to the warning, building a toilet away from the baby's bed, and using plastic drawers instead of metal drawers (26). In the quiet time protocol, it is important to identify the source of the patient's stress as an environmental stimulus, prevent environmental stressors, and increase adaptation to the environment. Quiet periods are implemented along with the modification of nursing assessments, care activities, and treatment methods to improve the patient's adaptation to the environment and improve the patient's sleep quality (27).
Considering the increasing number of premature births and the long-term stay of premature neonates in NICUs, attention should be paid to the care that shortens this procedure while being harmless, affordable, and effective. Meanwhile, it is necessary to reduce tension in premature babies because the repetition of tension and stress is associated with harmful effects on their neurodevelopment (9). Therefore, caregivers of neonates should pay attention to improving the physiological parameters of neonates because the main goal of admitting preterm infants to the NICU is helping to improve and stabilize physiological parameters. Fluctuations in temperature, flexible chest, undeveloped lungs, and breathing regulation center make premature infants unable to breathe effectively. Apnea occurs as a result of the intensification of periodic breathing and hypoventilation. Consequently, preterm infants are prone to many physiological disorders, such as bradycardia, hypotension, cardiac disorders, and apnea (28). A very low or high heart rate can indicate disease, infection, or pain, and abnormal respiratory rates are often associated with hypoxemia, hypercapnia, or acidosis (29). Therefore, arterial blood oxygen saturation (SaO2) percentage, breathing rate, and heart rate are three important physiological indicators (30). One of the interventions that can be effective in this field is the quiet time protocol, which limits the two mentioned stimuli to some extent. Therefore, the present study was conducted to investigate the effect of the quiet time protocol intervention on the physiological parameters of premature neonates admitted to the NICU of Ali Ibn Abi Taleb Hospital in Zahedan, Iran, in 2022.
2. Objectives
The present study aimed to evaluate the impact of the quiet time protocol intervention on the physiological parameters of premature neonates admitted to the NICU of Ali Ibn Abi Taleb Hospital in Zahedan, Iran, in 2022.
3. Methods
This quasi-experimental study was conducted on 62 premature neonates admitted to the NICU of Ali Ibn Abi Taleb Hospital in Zahedan, Iran, in 2022.
A convenient sampling method was employed to select the eligible cases. The inclusion criteria entailed being 34 - 36 weeks of gestational age, stability of neonatal physiological parameters, absence of severe respiratory distress, not using narcotic drugs or sedatives for the neonate, Apgar score of 8 - 10, obtaining a written consent form from the neonate’s parents, absence of congenital anomalies, low birth weight (birth weight 1500 - 2499 gr), and breastfeeding of the neonate. The exclusion criteria included neonate's seizures, neonates discharge, changes in the clinical condition of the neonates, such as intubation and hemodynamic disorder during the study (heart rate more than 180 or less than 100 times per min, breathing more than 60 or less than 30 times per min, and decrease in SaO2 percentage less than 85).
The data collection tool consisted of a demographic data form designed by the researcher based on previous similar studies containing gestational age, gender, Apgar minutes 1 and 5, number of hospitalization days, weight, respiratory rate, heart rate, SaO2 percentage of the neonate, as well as the average sound and light of the ward before, during, and after the intervention. The validity of the mentioned tool was investigated by applying the content validity method. To check the reliability of the tool, the equivalent reliability method was used, which was monitored by two people at the same time. In this way, the SaO2 percentage, respiratory rate, and heart rate of ten neonates were collected by two observers (researcher and trained nurse) separately from the monitor. The reliability was obtained through the correlation coefficient test, which was equal to 100%, and the reliability equivalent was used to assess the reliability of the monitor so that each time before the intervention, their accuracy was compared with another device.
After obtaining authorization from the faculty and the Ethics Committee of Zahedan University of Medical Sciences (code: IR.ZAUMS.REC.1400.354) and giving the necessary explanations, and obtaining permission, the researcher visited the NICU daily. The samples were collected after explaining the purpose of the research and the characteristics of the samples to the head nurse. Employing convenient sampling, the samples that were eligible to be included in the study were selected out of the neonates admitted to the NICU of Ali Ibn Abi Taleb Hospital in Zahedan, Iran. First, the researcher started collecting the data from the control group samples in 60 min during the evening shift from 15 to 17 o’clock to reach the required number of participants. Afterward, she collected the data of the subjects in the intervention group.
The physiological parameters of each neonate were evaluated for 60 min during the evening shift of 15 - 17 o’clock because the effect of the intervention is short-term and specific to the time of the intervention (21). In the intervention group, the personnel and the neonate's companion were informed before the intervention, and drug therapy, diaper change, neonate's necessary supplies, and positioning of the baby in the fetal position were all completed. All neonates were fed at the same time intervals pre-intervention. Next, the researcher washed her hands and connected the pulse oximetry to the neonate's hand to evaluate the physiological parameters. In this way, the neonate was monitored in terms of physiological parameters during the study. Sound and light were measured simultaneously by Sound Meter and Lux Meter software and were recorded every 20 min.
In the pre-intervention phase, the intervention group was examined for 20 min (from 3:00 until 3:20 p.m.) without implementing the quiet time protocol intervention, and the information was recorded in the demographic information form. Afterward, to implement the quiet time protocol, the neonate was moved to a separate room, and the researcher started the intervention by installing a sheet with the title 'please close the door quietly' on the entrance door of the ward. Moreover, ward lighting, neonate manipulation, movements, and conversations were reduced. Mobile phones were set on silent mode, the alarms of devices connected to the patient were set to a minimum level, and the ward computer was turned off if not needed (due to the fan noise). Furthermore, quick responses were sent to warnings, chair moving was prohibited in the nursing station, and an incubator cover was used for 20 min (from 15:30 to 15:50) (28, 29). During this period, the researcher recorded the neonate's physiological parameters in the instrument table as in the previous step. In the last stage, the neonate was observed by the researcher in the incubator for 20 min (from 16:00 until 16:20), and his physiological parameters were recorded as in the previous stages. All the procedures performed for the intervention group were implemented for the neonates of the control group as well. The difference was that the quiet time protocol was not performed for the neonates in the control group. In the end, the researcher and the researcher's assistant interpreted and completed the form.
3.1. Data Analysis
Data were analyzed using descriptive and analytical statistics. The statistical indicators in the descriptive statistics section included number, percentage, mean, standard deviation, maximum, and minimum. In the analytical statistics section, the chi-squared test, repeated measures analysis of variance (ANOVA), independent t-test, and if necessary, the Bonferroni post hoc analysis were used. The significance level in this study was considered lower than 0.05. The SPSS software version 22 was used to analyze the data. In addition, informed consent was obtained from all participants, and they were assured of confidentiality.
4. Results
The results of the Shapiro-Wilk test showed that the research data had a normal distribution. Therefore, parametric tests were used for data analysis. According to the results, the average gestational age, weight, and Apgar score at the first minute had no statistically significant differences between the premature neonates in the intervention and control groups. However, the number of hospitalization days was significantly different between the two groups (Table 1).
| Demographic Variables | Groups | P-Value b | |
|---|---|---|---|
| Intervention N = 31 | Control N = 31 | ||
| Gender | 0.79 | ||
| Male | 18 (58.1) | 17 (54.8) | |
| Female | 13 (41.9) | 14 (45.2) | |
| Diagnosis | 0.21 | ||
| Prematurity | 10 (32.2) | 5 (16.1) | |
| RDS | 11 (35.5) | 15 (48.4) | |
| Sepsis | 10 (32.3) | 11 (35.5) | |
a Values are expressed as No. (%).
b Statistical test: Chi-square test.
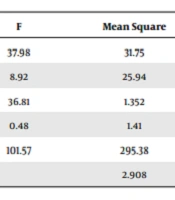
The frequency of gender and diagnosis showed no statistically significant differences between the premature neonates in the intervention and control groups (Table 2). The independent t-test revealed that the two groups pre-intervention (P < 0.001) and post-intervention (P = 0.04) had a statistically significant difference in terms of the average percentage of SaO2, but the difference during the intervention was not statistically significant (P = 0.75) (Table 3). There was also a significant difference between the groups in the effect of the intervention (P = 0.004), which means that the intervention has changed the SaO2 percentage. In terms of the interaction of time and group, there was no significant difference (P = 0.2). In other words, the changes in SaO2 percentage were not the same in the two groups, and the changes in the score of the intervention group were more than in the control group. Also, the effect of the pre-test score was statistically significant (P < 0.001) so number of the hospitalization days was effective on the results (Table 4).
| Demographic Variables | Groups | P-Value b | |
|---|---|---|---|
| Intervention | Control | ||
| Gestational age (wk) | 34.70 ± 0.78 | 34.83 ± 0.86 | 0.53 |
| Weight (gr) | 2043.22 ± 320.44 | 2081.77 ± 298.76 | 0.62 |
| Number of days of hospitalization | 2.41 ± 2.07 | 3.77 ± 3.02 | 0.04 |
| Apgar score one minute | 8.77 ± 0.42 | 8.22 ± 0.42 | 0.23 |
a Values are expressed as mean ± SD.
b Statistical test: t-test.
| Variable SaO2 | Groups | P-Value b | |
|---|---|---|---|
| Intervention | Control | ||
| Before intervention | 92.87 ± 3.49 | 95.41 ± 1.66 | < 0.001 |
| During intervention | 95.58 ± 2.34 | 95.41 ± 1.66 | 0.75 |
| After intervention | 94.25 ± 2.71 | 95.41 ± 1.66 | 0.04 |
a Values are expressed as mean ± SD.
b Statistical test: t-test.
| Variables | F | Mean Square | df | Sum of Squares | P-Value |
|---|---|---|---|---|---|
| Time | 37.98 | 31.75 | 1 | 31.75 | < 0.001 |
| Group | 8.92 | 25.94 | 1 | 25.94 | 0.004 |
| Time and group | 36.81 | 1.352 | 1 | 1.352 | 0.2 |
| Days of hospitalization (covariate) | 0.48 | 1.41 | 1 | 1.41 | 0.48 |
| Blood oxygen before (covariate) | 101.57 | 295.38 | 1 | 295.38 | < 0.001 |
| Error | 2.908 | 58 | 168.673 |
Regarding the average heart rate, the independent t-test showed that the two groups pre-intervention (P = 0.67) and post-intervention (P = 0.68) did not have a statistically significant difference. However, the two groups showed a statistically significant difference in terms of the average heart rate during the intervention (P < 0.001) (Table 5). The results of repeated measures ANOVA regarding heart rate, with the number of hospitalization days as a covariate, showed that changes in heart rate over time were significant (P < 0.001). However, no significant difference was observed in the effect of the intervention (P = 0.17), which means that the intervention did not change the heart rate. In terms of the interaction effect of time and group, there was no significant difference (P = 0.05) (Table 6). Although the average heart rate decreased during the intervention, the repeated measures ANOVA showed that the intervention did not impose an effect.
| Variable HR | Groups | P-Value b | |
|---|---|---|---|
| Intervention | Control | ||
| Pre-intervention | 148.19 ± 12.91 | 146.67 ± 15.43 | 0.67 |
| During intervention | 135.87 ± 16.02 | 146.67 ± 15.43 | < 0.001 |
| Post-intervention | 145.12 ± 14.74 | 146.67 ± 15.43 | 0.68 |
a Values are expressed as mean ± SD.
b Statistical test: t-test.
| Variable | F | Mean Square | df | Sum of Squares | P-Value |
|---|---|---|---|---|---|
| Time | 1009.496 | 170137.204 | 2 | 170137.204 | < 0.001 |
| Group | 1.875 | 733.58 | 1 | 733.58 | 0.17 |
| Time and group | 7.213 | 1215.661 | 2 | 1215.661 | 0.05 |
| Days of hospitalization group (covariate) | 0.02 | 7.874 | 1 | 7.874 | 0.88 |
| Error | 391.143 | 59 | 23077.438 |
The independent t-test showed that the two groups did not have a statistically significant difference in terms of the average respiratory rate pre-intervention (P = 0.64) and post-intervention (P = 0.29). However, the two groups were significantly different in terms of the average respiratory rate during the intervention (P = 0.01) (Table 7). The results of repeated measures ANOVA regarding respiratory rate, with the number of hospitalization days as a covariate, indicated that changes in respiration over time were significant (P < 0.001) (Table 8). There was no significant difference between the groups in the effect of intervention (P = 0.24), meaning that the intervention did not change the respiratory rate. In terms of the interaction effect of time and group, there was no significant difference between the two groups (P = 0.05). Although the average respiratory rate decreased during the intervention, the repeated measures ANOVA demonstrated that the intervention did not exert a significant effect.
| Variable RR | Groups | P-Value b | |
|---|---|---|---|
| Intervention | Control | ||
| Pre-intervention | 45.41 ± 9.71 | 46.61 ± 10.57 | 0.64 |
| During intervention | 40.09 ± 9.22 | 46.61 ± 10.57 | 0.01 |
| Post-intervention | 43.90 ± 9.58 | 46.61 ± 10.57 | 0.29 |
a Values are expressed as mean ± SD.
b Statistical test: t-test.
| Variable | F | Mean Square | df | Sum of Squares | P-Value |
|---|---|---|---|---|---|
| Time | 17.005 | 101.08 | 1 | 101.08 | < 0.001 |
| Group | 1.370 | 406.53 | 1 | 406.53 | 0.24 |
| Time and group | 32.71 | 194.48 | 1 | 194.482 | 0.05 |
| Days of hospitalization group (covariate) | 0.38 | 113.55 | 1 | 113.55 | 0.53 |
| Error | 296.831 | 59 | 17513.031 |
5. Discussion
Based on the findings of the present study, the two groups had a statistically significant difference in terms of the average SaO2 percentage. Moreover, the changes in the SaO2 percentage over time were significant, there was a significant difference in the intervention effect, and repeated measures ANOVA showed that the intervention had the desired effect on the SaO2 percentage. The findings of the current research in terms of applying the quiet time protocol on the physiological parameters of premature infants are consistent with the results of other studies. Abdeyazdan et al. investigated the impact of using earmuffs on the physiological and motor responses of premature infants. Their findings revealed that when infants used earmuffs, the average arterial oxygen saturation was significantly higher and their motor response was lower (30). Therefore, their results are consistent with the present study. Zeraati et al. evaluated the effect of quiet time protocol on the SaO2 and respiratory rate of premature infants. In the intervention group, the quiet time protocol (restriction of sound, light, and manipulation of the infant) was implemented for 16 - 18 hours, while the control group received a routine program for 11 - 13 hours. The findings of the research showed that the percentage of oxygen saturation in the quiet time group in the first, second, and third hours during the intervention was not significantly different, which is not in line with the present study and could be due to the unpredictability of some sound stimuli during the implementation of the protocol. Hospitalization of most babies due to respiratory distress syndrome may also play a role in not increasing the percentage of SaO2 (31). Therefore, the results of this research are not consistent with the present study, which could be attributed to the unpredictability of some sound stimuli during protocol implementation. Hospitalization of most babies due to respiratory distress syndrome can also be involved in not raising the percentage of SaO2.
Comparing the average heart rate of the two groups before and after the intervention, there was no statistically significant difference, but the two groups were significantly different in terms of the average heart rate during the intervention. In other words, although the average heart rate decreased during the intervention, repeated measures ANOVA revealed that the intervention did not have a significant effect. The findings of the present study are not consistent with the results of other studies concerning the influence of the quiet time protocol on the physiological parameters of premature infants. Kargar et al. investigated the impact of the sound control program on some physiological parameters of premature babies and the level of sound intensity in the NICU. Their findings showed that the average intensity of sounds and its maximum and minimum pre-intervention in three shifts were higher than standard. No significant difference was observed in the percentage of SaO2, heart rate, and their changes before and after the intervention (32), which is consistent with the results of the present study.
The two groups were not significantly different in terms of the average respiratory rate pre- and post-intervention, while a statistically significant difference was observed in the average respiratory rate during the intervention. The indifferent post-intervention results can be because the effect of the intervention is not permanent. Although the average respiratory rate declined during the implementation of the intervention, the repeated measures ANOVA shows that the intervention did not have a significant effect. The findings of the present study concerning the influence of the quiet time protocol on the physiological parameters of premature infants are not consistent with similar studies. Kargar et al. assessed the impact of the sound control program on some physiological parameters of premature babies and the level of sound intensity in the NICU. Their findings showed that the average intensity of sounds and their maximum and minimum pre-intervention in three shifts were higher than the standard. No significant difference was observed in the percentage of SaO2, heart rate, and their changes before and after the intervention (32), which is consistent with our results. Nasimi et al. determined the effect of the quiet time protocol on the physiological parameters of premature infants. Their findings indicated that the average heart rate and respiration of the intervention group infants in the second hour of the intervention were significantly lower than the control group infants (21), which is not in line with the results of the present study. This discrepancy may result from the unpredictability of some sound stimuli during the implementation of the protocol and the different conditions of babies from one person to another.
5.1. Conclusions
The results of the current research showed that with the implementation of the quiet time protocol, the changes in SaO2 percentage were not the same in the two groups, and the changes in the score of the intervention group were higher than the control group. The average heart rate and the respiratory rate decreased during the intervention, but the intervention was not effective on the heart rate and respiratory rate. Considering the effectiveness of the quiet time protocol to reduce environmental stimuli and the non-destructive effect on the physiological parameters of the infant, this method is useful for environmental and behavioral changes. These measures are recommended as standard care measures to reduce stress and improve the growth and development of premature neonates in the NICU.
5.2. Limitations
Among the limitations of this study, we can point out the lack of control over environmental stimuli, such as extra sounds and light. In order to further control the said stimuli, the infants were transferred to a separate room for the intervention.