1. Context
Infants admitted to the neonatal intensive care unit (NICU) often require intravenous access to medication, fluids, and nutrition (1, 2). As neonates’ veins are small and fragile (3), peripheral venous catheter placement is associated with complications such as extravasation, infiltration, phlebitis, leakage, spontaneous displacement, and catheter-related bloodstream infection (CRBSI) (1). Extravasation is a serious complication (4) and consists one of the important causes of death in infants (5). Extravasation is the penetration of intravenous fluid into the surrounding tissue, the physiology of which is not yet fully understood. Still, there are two main theories about its etiology: (1) catheter displacement; or (2) increased vascular permeability (1). The most common cause of extravasation injury in infants is injection feeding. Other causes include injectable solutions such as dextrose and calcium, blood, platelets, sodium bicarbonate, total solution (tromethamine), a combination of dextrose and flucloxacillin, insulin and aminophylline, dextrose, and magnesium, all stimulating the venous endothelium and increasing the risk of venous rupture (6). Data on the incidence of extravasation injuries vary considerably, as definitions and documentation are inconsistent, and no reliable documentation exists (7). Extravasation injury has been reported in different studies from 18 to 70% (3, 8, 9). Atay et al. reviewed 452 peripheral vein catheters implanted for infants and observed 45.6% extravasation/infiltration (9). Furthermore, in a retrospective analysis of 42 children, 41 extravasation injuries (98%) involved peripheral intravenous catheters, and only one case (2%) involved central intravenous catheters. In 40 cases, the organs were affected, and in only 1 case, the scalp was affected (10). While many minor injuries resolve spontaneously and do not cause tissue necrosis, some cases lead to serious complications (1). Even in some cases, serious complications such as pain, necrosis, tendon and nerve damage, compartment syndrome, scar, limb movement limitation, or even amputation may occur depending on the injury’s type, extent, and location (7). About 4% of neonates leave the NICU with visible scars from extravasation injury (7).
Extravasation is an emergency (11), and the resulting injury requires immediate care (12, 13). Treatment approaches mainly include elevation of the limb, temperature management (hot or cold compresses), medications, wound care products, and surgical procedures (14). In a study on 34 cases of extravasation injury in the neonatal intensive care unit, only six necrosis cases were recorded after immediate intervention (within 30 minutes) (5). Moreover, a study by Odom et al. on 147 newborns and children showed that initial treatments, including removing the venous catheter, using warm compresses, elevating the limbs, using cold compresses or a combination of these treatments, and injecting hyaluronidase or phentolamine were effective, none needed surgical intervention for wound healing and none developed compartment syndrome or wound infection (14), suggesting that extravasation injury requires structured evaluation and treatment.
However, the published results of randomized and controlled clinical trials are contradictory (11), and there are no agreed guidelines on managing extravasation injuries in infants (7). This is mainly because many published studies are of limited value in aiding treatment decisions (12). Nevertheless, extravasation damage is preventable. Equipping nurses with the necessary knowledge and skills of intravenous treatments in infants is essential for preventing early diagnosis and managing extravasation injuries (15). Considering the researcher’s experience as a NICU nurse and the significance of extravasation injury management, this study aimed to review clinical trials conducted on extravasation injury care in infants.
2. Procedure
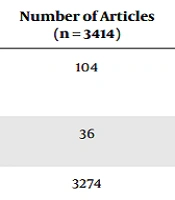
The related articles were searched in ProQuest, Magiran, PubMed, and SID databases, using the terms “extravasation” OR “vascular leakage” OR “Peripheral Infiltration” AND “Neonatal” OR “Newborn” OR “Infants” OR “NICU” in the titles and abstracts of the articles by two researchers independently. Articles published in English from January 2000 to December 2022 were searched. At first, only clinical trial and quasi-experimental studies were searched, followed by review articles, original articles, case series, and case reports. Comments, cadaver studies, and animal experiments were removed. The full text of the articles was studied, and the most relevant articles were included in the final analysis. The details of the search strategy and the number of extracted articles are given in Table 1:
| Database | Keywords | Number of Articles (n = 3414) |
|---|---|---|
| PubMed | (“extravasation”[TIAB] OR “vascular leakage”[TIAB] OR “Peripheral Infiltration”[TIAB]) AND (“Neonatal” [TIAB] OR “Newborn” [TIAB] OR “Infants” [TIAB] OR “NICU” [TIAB]). Filters applied: Full text, Case Reports, Clinical Study, Clinical Trial, Randomized Controlled Trial, Review, Systematic Review, English, from 2000/1/1 - 2023/1/1. | 104 |
| ProQuest | Abstract ((“extravasation” OR “vascular leakage” OR “Peripheral Infiltration”)) AND abstract ((“Neonatal” OR “Newborn” OR “Infants” OR “NICU”)) | 36 |
| Scopus | (“extravasation” OR “vascular leakage” OR “Peripheral Infiltration”) AND (“Neonatal” OR “Newborn” OR “Infants” OR “NICU”) | 3274 |
Abbreviation: NICU, neonatal intensive care unit.
3. Results
As shown in Table 1, 3414 articles were extracted from the databases. After removing duplicates, the title and abstract of the articles were searched, and after removing unrelated articles, 132 related studies were selected, and their full texts were analyzed. Given the unavailability of some databases, the full texts of 8 articles could not be found. Finally, after reading the full text of the available articles, 20 studies, including nine case reports, five case series studies, three clinical trial studies, and three review articles, entered the final analysis stage. The results showed that the average fetal age reported in these studies was 32 weeks, the average age was 11 days, and the average weight was 2240 grams. Moreover, 57% of boys and 1% of girls experienced extravasation. In addition, most extravasation-related drugs were hypertonic dextrose serum, calcium, TPN, and dopamine. Concerning the organs involved, the results showed that 46% of the cases were related to the upper limbs (including arms, hands, and forearms), and 54% were related to the lower limbs (back and ankles). The data also indicated that various treatment methods are available for extravasation, including elevating limbs, pain relief, hot and cold compresses, flush-out technique with saline, dressings (hydrocolloid, hydrogel, and hydrocellular foam), drugs (2% nitroglycerin ointment and subcutaneous administration of hyaluronidase and phentolamine), new methods such as the application of amniotic membrane, omega-3-rich fish skin (Kerecis), active leptospermum honey (ALH), PRP, and surgical removal as detailed in Table 2:
| Row | Authors | Year | Country | Research Design | Sample | Affected Area | Materials Leading to Extravasation | Intervention (s) | Outcome(s) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ahn and Park (16) | 2021 | Korea | Case report | A male infant; fetal age: 40 weeks; birth weight: 3480 grams | Right side following calcium injection from the femoral vein | Calcium | Disinfectant ointment; hydrocellular foam dressing; wound debridement and then fish skin rich in omega-3 (Kerecis a) | After two months, the wound area was completely replaced by normal, healthy skin. No signs of infection were observed during treatment. |
| 2 | Altan et al. (17) | 2013 | Turkey | Case report | A 23-day-old female infant (no data about fetal age and weight) | Left forearm | Contrast agent | Elevating the upper limb and applying a cold compress; surgery (fasciotomy) | Improvement of pressure caused by compartment syndrome after surgery. The functional and neurological examinations showed no abnormality in the range of motion. |
| 3 | Boyar et al. (18) | 2014 | USA | Case series | Three infants; a case of extravasation in a 21-day-old girl with a fetal age of 26 weeks | Right ankle | Not specified | Medihoney gel (leptospermum honey) was started on the second day and covered with a hydrocolloid dressing. | The discomfort and edema around the wound decreased, and the wound healed after 3 weeks. |
| 4 | Cho et al. (19) | 2019 | Korea | Case series | Five infants; average fetal age: 30 weeks; average age: 26 days | Leg area: 4 cases; forearm: 1 case | Intralipid 10%; full intravenous feeding 12.5% | Dressing using a thick layer of Terramycin eye antibacterial ointment, sesame oil, and an anti-inflammatory herbal mixture covered with Vaseline and wet gauze after rinsing with normal saline | Within 20 to 50 days, the wounds were completely healed. |
| 5 | Odom et al. (14) | 2018 | USA | Retrospective study | 147 infants and children; aged 3 days to 14 years; 59% boys and 41% girls; (no data on the number of babies and fetal age) | Lower limbs (26%); upper limbs (74%) | Not specified | Removing the venous catheter, using hot or cold compresses, elevating the limbs, and injecting hyaluronidase or phentolamine | Wound healing - no need for surgery - no infection in all patients |
| 6 | Sagheb et al. (20) | 2022 | Iran | Clinical trial | 16 infants (10 boys and 6 girls); average fetal age: 32 weeks; average weight: 1370 grams; age range 7 - 29 days | 11 hands; 5 cases of the end of the lower limb | Calcium chloride; intralipid; dextrose 12.5% | Injection of hyaluronidase and warm compresses in the first 24 hours/change of dressing by washing with normal saline and use of fibrinolysin ointment on the wound and then dressing with phenytoin ointment until recovery | Completing the healing process of the lesions without surgery and discharging the infants without any complications. Average recovery time: 13 days |
| 7 | Lee et al. (21) | 2013 | Korea | Case report | One-month-old male infant; fetal age: 24 weeks; birth weight: 470 grams | The outer side of the right foot | Sodium bicarbonate | Administering hyaluronidase in the subcutaneous tissue; local use of epithelial growth factor and foam dressing for up to 3 weeks; treatment with local PRP prepared from the mother’s peripheral blood (0.3 mL of PRP daily on the wound bed) | In the first 3 weeks, the treatments were ineffective and there was extensive necrosis, but four weeks after PRP treatment, the wound was completely healed, and there was no limitation in range of motion. |
| 8 | Subhani et al. (22) | 2001 | USA | Case report | One-day term infant; birth weight: 3340 | Left foot | Dopamine | Subcutaneous administration of phentolamine | Improvement of dopamine-induced cutaneous ischemia after phentolamine injection |
| 9 | Harris et al. (23) | 2001 | England | Retrospective; review | 56 infants (no background available) | Not specified | Dextrose, calcium, potassium, bicarbonate, inotropes, TPN | Washing with saline | No loss of skin integrity and need for surgical reconstruction |
| 10 | Yew et al. (13) | 2022 | Malaysia | Case series | 3 babies: A 36-week and 2-day-old girl; a 38-week and 1-day girl; a 37-week and 4-day boy | An arm; two cases back of the hand | TPN; albumin; dextrose 10% | Flush-out technique with normal saline | Wound healing without scarring |
| 11 | Casanova et al. (24) | 2001 | France | Case series | 14 babies: Average age: 20 days; average weight: 2490 grams; 6 pre-term babies and 8 full-term babies | 9 feet; 3 hands; 1 elbow; 1 forehead | Caffeine, beta blocker, dopamine, calcium | 12 cases with aspiration and hyaluronidase infiltration, 1 case with saline aspiration and infiltration, and 1 case with hyaluronidase infiltration | Three infants developed necrosis, but eventually, all infants fully recovered. |
| 12 | Kostogloudis et al. (5) | 2015 | Greece | Retrospective; review | 34 babies with average fetal age: 32 weeks; average weight: 1885 grams; average age: 11 days | Not specified | Not specified | First, piercing the area and washing it with saline, then dressing in gauze soaked in paraffin and a layer of gauze soaked in povidone-iodine | In 21 infants, there were no signs of soft tissue damage up to 24 hours after treatment. The ischemic symptoms in 6 patients decreased within 25 days. |
| 13 | Kuensting (25) | 2010 | USA | Case report | 4-day-old male infant; fetal age: 37 weeks; birth weight: 3000 grams | Right foot | Dextrose 10% | Hyaluronidase infiltration | Wound healing completed within 8 days |
| 14 | Siu et al. (26) | 2007 | China | Case report | 34-week infant; birth weight: 1520 grams | Back of the right foot | A mix of dextrose, calcium, potassium, and other ions | Hyaluronidase infiltration and washing with saline | Wound healing completed within 5 days |
| 15 | Mosalli et al. (27) | 2013 | Saudi Arabia | Case report | 1 female infant; fetal age: 25 weeks; birth weight: 520 grams | Right arm | Not specified | Elevating the limb; warm compresses and use of nitroglycerin ointment | Wound healing |
| 16 | Sawatzky-Dickson and Bodnaryk (28) | 2006 | Canada | Quasi-experimental (interventional) study | 9 infants; fetal age: 24 to 40 weeks; weight: 582 to 4.404 grams | 5 cases of the back of the foot; 4 cases of hands and forearm | Dextrose 10% with calcium/sodium/potassium; sodium bicarbonate; dense red blood cells; TPN | Washing the wound with normal saline and then using a blue gel, Hydrofiber sheet, and hydrocolloid dressing. | Healing of injuries caused by extravasation - none of the patients suffered from wound infection. |
| 17 | Reynolds (29) | 2007 | Scotland | Case report | Female infant; fetal age: 29 weeks | Back of the left hand | TPN and 10% dextrose mixed with calcium and sodium | Hyaluronidase infiltration and flushing of normal saline and aspiration of stimulants | Recovery completed after two weeks |
| 18 | Kadivar et al. (30) | 2023 | Iran | Single-arm clinical trial study | 3 male infants and 1 female infant; average fetal age: 33 weeks; average weight: 2255 grams | 3 feet | Blood; dextrose 10% and 12.5% | AM | Complete recovery and positive impact on the wound-healing process; the average recovery time was 12.5 days |
| 19 | Mohr et al. (31) | 2014 | USA | Case series | 3 infants; the average fetal age: 27 weeks | 2 feet and 1 forearm | Dopamine | Use of ALH | Wound healing in all three infants |
| 20 | Boyar and Galiczewski (32) | 2018 | USA | Case series | 4 infants; 3 boys with an average of 28 weeks; a 25-week-old girl | 1 foot and 3 hands and forearms | TPN; dextrose 10% | The use of dHAMA | Wound healing in all four infants; the skin at the injury site was not discolored, and a soft scar was barely visible. |
Abbreviations: AM, amniotic membrane; ALH, active leptospermum honey; dHAMA, dehydrated human amniotic membrane allograft.
a Kerecis is a biotechnology company in Iceland. It is pioneering the use of fish skin in the globally expanding regenerative medicine market.
4. Discussion
Clinically, the acute phase of extravasation is usually characterized by local pain and edema (33). Depending on the fluid and volume removed, manifestations include pain, swelling, erythema, tenderness, and localized blisters. Blisters and stiffness that persist for more than 24 hours indicate severe extravasation damage and the risk of ulceration (13). Capillary refill time may be prolonged, and the pulse may decrease (33). In any case, the first step is to stop the infusion or injection immediately. Further management should be planned before removing the IV cannula, depending on the severity of the injury and extravasated fluid. It is important to determine whether the fluid is irritant or vesicant, the presence of an antidote and whether it should be administered topically via an indwelling venous cannula. This is especially true for critically ill infants who cannot undergo immediate surgery or have received only a small amount of extravasation (34). The catheter should be removed as soon as possible during aspiration of any possible residual drug (14, 34). The extravasation interventions are discussed below:
4.1. Nitroglycerin Ointment 2%
A procedure that is used in some cases but not described in the literature is the use of nitroglycerin ointment. The primary action of nitroglycerin is to relax vascular smooth muscles and dilate post-capillary vessels. Peripheral vasodilatation caused by nitroglycerin may increase the absorption of infiltrating fluids, thereby minimizing tissue damage. The infant should also be monitored for hypotension and tachycardia, potential side effects of nitroglycerin (28). For vasoconstrictors (e.g., adrenaline, dopamine, and epinephrine) that can lead to ischemic necrosis through a vasopressor effect, topical application of 2% nitroglycerin as a dressing is effective for removed fluids that may cause peripheral tissue ischemia (12). However, absorption through the skin is associated with potential systemic effects such as hypotension and tachycardia (28). Thus, nitroglycerin should not be used on punctured or damaged skin and can only be used in children over 21 days old (35). The effect of topical nitroglycerin on extravasation wound healing in infants was demonstrated in a case report by Mosalli et al. in Saudi Arabia. This study was conducted on a 25-week-old girl with a birth weight of 520 grams (27). Furthermore, Baserga et al. demonstrated the positive effect of 2% nitroglycerin ointment in two infants with vasoconstriction-induced peripheral ischemia caused by umbilical artery catheterization (36).
4.2. Phentolamine
As a non-selective alpha-adrenergic receptor blocker, phentolamine can reduce tissue ischemia by dilating vessels and thus prevent ischemic necrosis (22, 37). Phentolamine is a therapeutic agent to prevent skin necrosis and lesions caused by vasoconstrictor drugs, including dopamine. Early diagnosis and prompt treatment of the affected area with an alpha-receptor antagonist such as phentolamine may prevent permanent damage and the need for a skin graft. Phentolamine is an alpha-adrenergic receptor-blocking agent that causes peripheral vasodilation (22). The effect of phentolamine is seen almost immediately, and the dose can be re-administered if necessary. The biological half-life of subcutaneous injection of phentolamine has been reported to be less than 20 minutes (38). After administering phentolamine, patients should be carefully monitored for blood pressure drops (22). In a retrospective study, Odom et al. showed that among 147 cases of extravasation, phentolamine was used in only one case, resulting in wound healing (14). Moreover, a case report by Subhani et al. showed that subcutaneous administration of phentolamine improved skin ischemia in a one-day-term infant who had extravasation in the left leg after receiving dopamine (22).
4.3. Hyaluronidase
Although the efficacy of hyaluronidase in preventing injury in IV injuries has not been demonstrated in randomized controlled trials in humans, it remains a recommended antidote (28). Several studies have shown the positive effect of hyaluronidase (150 U/mL solution) in managing extravasation in infants (14, 19, 20, 24, 25, 29). However, this drug is not recommended in infants less than 28 weeks because the skin of these infants is considered too premature (3). Notably, hyaluronidase should not be used to remove vasoconstrictor drugs (dopamine, adrenaline, noradrenaline, etc.) (19). Some researchers have suggested that subcutaneous injection of hyaluronidase should be used within the first hour after extravasation, which can reduce the severity of tissue damage (19, 39). In contrast, others suggested that this technique should be performed within six hours after the injury (24). A retrospective study in 2019 examined 36 infants with extravasation, in which 11 of the 36 patients underwent hyaluronidase injection. This intervention prevented the progression of the wounds to a necrotic state and significantly reduced the severity of the wounds (19). Casanova et al. studied 14 infants with extravasation injuries, of whom 12 were treated with aspiration and hyaluronidase infiltration, one with saline aspiration and infiltration, and one with hyaluronidase infiltration. Three infants developed necrosis, but eventually, all recovered completely (24). A review study in 2004 investigated 36 infants with extravasation over an 18-month follow-up. Subcutaneous administration of hyaluronidase and washing with normal saline were used in these infants. None of the infants required surgical debridement, and the wound healed in all (40). A retrospective review of 56 extravasation injuries over three years examined lavage with 500 mL of normal saline without hyaluronidase infiltration and reported excellent results, and none of these infants required reconstructive surgery (23). A retrospective study by Odom et al. showed that hyaluronidase was used for treatment in 25 cases of extravasation, and all showed the positive effect of hyaluronidase on wound healing. Although several studies have reported favorable results with subcutaneous injection of hyaluronidase, it should be used with great caution in infants, especially in those with renal insufficiency, due to the risk of overhydration (14). A case report by Lee et al. on a one-month-old boy who developed an ulcer on the right leg following sodium bicarbonate injection showed that subcutaneous injection of hyaluronidase and foam dressing failed to heal the infant’s wound (21).
4.4. Hot and Cold Compresses
There is a controversy over the use of hot and cold compresses, especially concerning eliminating stimulants and reducing agents (41). A cold compress may cause local vasoconstriction and reduce cellular damage. Thus, it can reduce the inflammatory reaction and further release of the substance in the tissue. However, the degradation of the substance and the healing process may also be delayed, potentially accelerating or worsening peripheral neuropathy. At the same time, a warm compress may increase drug removal by local vasodilation and prevent peripheral neuropathy. On the other hand, a warm compress can lead to faster metabolism and accelerated tissue damage (7). Thus, further research is needed in this area. In our search, only three studies addressed the use of hot and cold compresses in treating injury caused by extravasation. In their study in Iran, Sagheb et al. used hot compresses in 16 infants in the first 24 hours after extravasation injury and the subcutaneous administration of hyaluronidase. After 24 hours, they applied other managements (washing with normal saline, using fibrinolysin ointment, and dressing with phenytoin) (20). In a study in Turkey, Altan et al. used limb elevation and cold compress for a 23-day-old girl who was injured in the forearm after contrast injection (17). In a retrospective review, Odom et al. examined 147 infants and children. They reported that hot and cold compresses were used with other treatments (hyaluronidase, phentolamine) for injury caused by extravasation in infants (14).
4.5. Dressing
Special wound dressings, such as hydrocolloid or foam dressings, may be considered after extravasation and other interventions for tissue repair and wound infection prevention (12, 42, 43). In the acute phase of extravasation, dressing is usually considered a secondary intervention (7). Evidence has shown that hydrogel dressings are safe for preterm infants of all fetal ages. These dressings consist of 80 to 90% water and can be applied directly to the skin and keep the wound moist, which causes the destruction of necrotic tissues and increases the autolysis rate (3). Sawatzky-Dickson and Bodnaryk developed a protocol performed on nine infants with extravasation for one week. In this protocol, the wound was washed with normal saline, and then an aqueous gel, hydrofiber sheet, and hydrocolloid dressing were used. The results showed that this protocol was safe and improved extravasation injuries in infants, and none of the patients suffered wound infection. This protocol provided instructions for nursing staff (28). Moreover, a study in the United States in 2014 confirmed the effect of hydrocellular foam dressing on the wound of a 21-day-old female infant (18).
4.6. The Saline Flush-Out Technique
Two techniques, liposuction and flush out with saline, were proposed by Gault in 1993 on 96 patients to remove excess material at the site of skin damage caused by extravasation (44). Saline solution (0.9% sodium chloride) was repeatedly applied through puncture wounds or small knife cuts in the affected tissue to remove the fluid (5). In this technique, the fluid can be removed more intensively using hyaluronidase (24). Reynolds report on a 29-week-old female infant who developed left-hand extravasation 40 hours after birth and the initiation of TPN showed that treatment with hyaluronidase infiltration, normal saline flushing, and aspiration of skin irritants led to complete wound healing after two weeks (29). In addition, Kostogloudis et al. studied 34 extravasation infants in Greece after examining 1409 hospitalized infants over 24 months. The flush-out technique was used with saline without hyaluronidase in the first half hour after the injury. The first step was to remove the granule and aspirate the area. After administration of fentanyl for pain relief, full-thickness skin incisions (≤ 3 mm long) were made under sterile conditions, and normal saline (60 mL on average) was used to wash the area. A layer of gauze soaked in paraffin and a layer of gauze soaked in povidone-iodine were placed on the wound, and the affected limb was placed above the body surface for 24 hours. In all cases, daily dressing changes were evaluated until complete healing. The results of this study were evaluated positively. The advantage of this method is its being noninvasive and no need for general anesthesia. It also minimizes the need for surgery and has a beneficial effect on wound healing in infants. The authors emphasized that this method should be used immediately in the first hour after the injury, and a dressing with sterile gauze impregnated with paraffin should be used in addition to washing the wound with normal saline (5). Gopalakrishnan et al. reviewed 10 case reports and case series studies and showed the effect of saline with or without hyaluronidase on extravasation in infants. The data also showed that out of 237 infants, 234 cases were recovered completely (11). A report by Yew et al. on 3 infants showed that the flush-out technique with normal saline was effective in healing without scarring (13). Furthermore, a review study by Gopalakrishnan et al. searched clinical trial studies on the effect of washing with normal saline with or without hyaluronidase on the treatment of extravasation in infants and ultimately did not find any clinical trials in this field (11).
4.7. Active Leptospermum Honey
One of the wound management treatments is using ALH in necrotic, secretory, and infected tissues (44). A large part of the literature is focused on using ALH in the adult population (45, 46). The effect of ALH in infants was evaluated in a report by Mohr et al. on three extravasation cases with different degrees of damage. Although the use of ALH in adult care is well documented, this case study demonstrated its potential use in wound healing in these three infants (31). Moreover, Boyar et al. used MedihoneyTM gel (leptospermum honey) on the second day to treat a wound caused by extravasation in a 21-day-old girl. The result was that the discomfort and edema around the wound decreased, and the wound healed after three weeks (18). Several other retrospective studies and case reports showed its effect on infant wounds (47, 48), but since they were not related to extravasation, they were not reviewed in the present study. In this study, we found that no clinical trials had addressed the effect of leptospermum honey on the treatment of neonatal wounds.
4.8. Amniotic Membrane
Amniotic membrane (AM) has been used in medicine since the early 20th century. However, the use of new innovative biological methods in the neonatal population is in its infancy. There is limited information on neonatal wounds, treatments, and outcomes (32). This method was performed in a single-arm clinical trial in Iran by Kadivar et al. from February 2020 to April 2022. Infants injured due to extravasation during this period were included in the study, and ultimately, 6 infants were examined. In this method, the amniotic membrane covered the damaged area, and the wound was re-evaluated after 48 hours. Five days later, the previous amniotic membrane was removed and replaced with another one. Bandages were changed every 5 to 7 days until recovery. The mean recovery time was 12.5 days (10 - 20 days), and no adverse reactions were observed. All infants recovered completely without scarring. This preliminary report showed that AM is a safe and effective treatment for neonatal extravasation (30). Boyar and Galiczewski investigated four infants with extravasation injuries. They showed no change in wound healing after the use of collagenase ointment and active leptospermum honey. Still, after the commencement of the treatment with dehydrated human amniotic membrane allograft (dHAMA), a significant improvement was observed in the newborns’ wounds. This study also confirmed the effect of the amniotic membrane on the healing of damage caused by extravasation in infants (32). However, controlled trials with larger sample sizes are needed to reevaluate these findings and determine practical implications.
4.9. Omega-3-Rich Fish Skin
The effect of fish skin in treating extravasation injury in infants has been confirmed in only one case report. In Korea, Ahn and Park demonstrated the effectiveness of this method in a 40-week-old male infant (16). After receiving calcium from the right femoral vein, the infant had erythema and a wound on the right side. Abdominal ultrasound showed the accumulation of calcium deposits in the subcutaneous lesion of the flank. The infant was treated with antiseptic ointment and hydrocellular foam dressing and was discharged on the ninth day of hospitalization. The infant was then referred to the plastic surgery outpatient department and underwent debridement. Omega-3-rich acellular fish skin graft (Kerecis, Arlington) was used on the injury site. Omega-3-rich fish skin was cut according to the size of the wound, soaked in 0.9% normal saline for 1 minute for hydration, and covered with hydrocellular foam. No signs of infection were observed during treatment. After two months, the defect was completely covered by normal healthy skin. There was no indentation or contraction at the wound site. There was only pigmentation in the scar.
4.10. Limitations of the Study
The main limitation of this study was the unavailability of some databases and the inaccessibility of the full text of some articles. Thus, it is necessary to search other databases in the next reviews.
5. Conclusions
The present study showed that there is no clear and comprehensive approach to the management of extravasation injury in infants. The ideal approach to injuries caused by extravasation is to prevent them in the first place. The next best step seems to be to reduce the damage by antidote or use of hyaluronidase. Substances for which there is no antidote cause more harm in infants. Thus, hyaluronidase remains the treatment of choice. Moreover, the saline technique described by Gault (44) seems a suitable option to remove the solution and minimize damage physically. More studies are needed to investigate the advantages and disadvantages of other methods.