1. Background
Surgical site infection (SSI) is the most common complication after surgical procedures such as cesarean sections (1). The prevalence of infection in women who undergo cesarean sections is 5 to 20 times higher than that of women who have normal deliveries (2). A cesarean section, the delivery of a fetus by cutting the abdominal wall (laparotomy) and uterine wall (hysterectomy) (3), has increased by 25% in the last decade as reported by the World Health Organization (4). While the American Health Care Association (AHCA) reported the ideal cesarean section rate to be 10 to 15% for countries, the cesarean section rate in Iran is far beyond the standard levels. According to the Health Department of the Iranian Ministry of Health, in 2021, 49% of births were delivered by cesarean section (5). Cesarean sections are associated with short-term and long-term complications, including maternal death (6), thromboembolism (7), bleeding (8), endometriosis (9), accidental surgical injuries (10), prolonged hospitalization (11-13), emergency hysterectomy (14), pain (15), and wound infection (16).
Cesarean section, as the most common surgical procedure in women, is associated with a high rate of SSIs (15 - 20%) (17, 18). In addition to the mentioned complications, SSIs can lead to endometritis, fever, urinary infections, microbial resistance, septic shock, prolonged hospital stays, higher treatment costs, and patient dissatisfaction (19, 20). However, about 40 to 60% of postoperative wound infections can be prevented (21, 22). Nurses in surgical departments and members of the surgical team in the operating room can prevent the occurrence of SSIs by fully complying with public health and sterilization principles and controlling factors effective in transmitting infection in preoperative, intraoperative, and postoperative stages. Adequate resources, comprehensive support by hospital managers, the knowledge and skills of surgical teams, thorough patient preparation, monitoring of patients after transfer from the operating room to the surgical department, and nursing support at the time of discharge may lead to a significant reduction in SSIs, reducing the death rate and saving economic resources (21).
New guidelines published by the WHO and Centers for Disease Control and Prevention (CDC) suggest some actions to reduce the risk of SSIs, including body washing with or without chlorhexidine gluconate before surgery, decolonization with mupirocin ointment to prevent Staphylococcus aureus infection in surgical candidates (especially cardiac and orthopedic surgeries), avoiding the removal of excess hair and, if necessary, removal with an electric shaver, preparing the skin of the surgical site in the operating room with alcohol-based antiseptics, prescribing a dose of first- or second-generation cephalosporin before surgery (maximum 120 minutes before making a skin incision, taking into account the half-life of the antibiotic), and organ support during the operation with normothermia, hyperoxygenation, and accurate control of the patient's blood sugar (20). In addition to the actions described above, that can be effective in preventing wound infection one of the important strategies to prevent surgical wound infection is the use of care bundles. A care bundle is a set of interventions that, when used together, significantly improve patient outcomes. A care bundle can be used to manage a specific condition, and the components in a bundle are evidence-based best care practices (23). A care bundle consists of a group of interventions, usually three to five evidence-based interventions, that are relevant to a specific condition or event in patient care. Interventions are grouped and conducted together to achieve more effective outcomes compared to when they are implemented separately (24). The purpose of providing care bundles is to ensure that all patients always receive the best care and treatment. When performed collectively and correctly, the care bundle improves patient outcomes (25). Studies have shown that implementing a care bundle involving evidence-based interventions is associated with a significant reduction in postoperative infections (26). Johnson et al. showed that adopting a care bundle approach can lead to a reduction in SSIs after hemiarthroplasty (27). Singh et al. investigated the usefulness of bundled interventions in reducing the incidence of surgical site infections in gynecological surgery and stated that the application of the care bundle is easy and possible in all stages and, in addition to reducing the risk of SSI, adds only three minutes to the total duration of surgery (28).
Given that cesarean incision site infection, after bleeding, is the most common complication after cesarean sections and is the third cause of maternal mortality, especially in developing countries, leading to increased hospitalization and treatment costs (29), designing and using a care bundle in patients undergoing cesarean sections may reduce the occurrence of this complication. Each hospital has the opportunity to create its cesarean section care bundle to reduce surgical site infection (30). However, despite the announcement of global guidelines for the prevention of surgical site infection (SSI) published in 2016 by WHO and CDC and evidence-based studies, there is still no effective protocol in accordance with the type of surgery and target group of patients in most medical centers. Thus, it is possible to prevent the occurrence of surgical site infection in women undergoing cesarean sections by designing a local and applicable preventive nursing care bundle, suitable for medical settings and target groups.
2. Objectives
The present study aimed to examine the effectiveness of the care bundle for the prevention of cesarean section wound infection in women admitted to Ali Ibne Abitalib Hospital affiliated with Zahedan University of Medical Sciences in 2022.
3. Methods
The protocol for this two-group blinded controlled randomized clinical trial study was confirmed with the clinical trial code IRCT20170703034873N3 and the code of ethics IR.ZAUMS.REC.1401.176 by the Vice-Chancellor for Research and Technology of Zahedan University of Medical Sciences. The participants were selected from all pregnant women candidates for non-emergency cesarean section who underwent cesarean surgery in the operating room of Ali Ibne Abitalib Hospital affiliated with Zahedan University of Medical Sciences. The inclusion criteria were non-emergency cesarean section candidates with obstetric and non-obstetric indications, at least 18 years of age, singleton pregnancy, gestational age greater than or equal to 37 weeks, absence of underlying diseases such as liver/severe kidney disorders/diabetes, and severe anemia (Hct < 33% or Hb < 11g/dL), not receiving blood products within 10 days before surgery, not using immunosuppressive drugs (cortones), no history of infectious diseases and severe malnutrition in the last 6 months, BMI less than 35, and not detecting placenta accreta or placenta previa in ultrasound before cesarean section. The exclusion criteria were not being discharged in the first 24 hours after surgery due to any birth complications and hospitalization for more than 1 week after cesarean section, fever above 38 degrees from the time of admission to 48 hours after cesarean section, and the patient’s non-referral in the one-month follow-up.
Taking into account the severity of surgical wound infection in a similar study (Alizadeh et al.), (31), the sample size was estimated with a 95% confidence interval and 95% test power as 19 persons per group using the following formula. To ensure sample adequacy for data analysis and considering any possible dropout, the sample size was considered to be 30 patients in each group (60 patients in total).
Z1-α/2 = 1.96; P1 = 1.8; P2 = 3.6; Z1-β = 1.64
The participants were selected from among pregnant women candidates for non-emergency cesarean section who visited the Obstetrics and Gynecology Department of Ali Ibne Abitalib Hospital using convenience sampling in autumn 2022. The selected women received some instructions about the objectives of the study and the research protocol and signed an informed consent form. The patients were assigned to the intervention and control groups using random permuted blocks. The data were collected using a checklist for gathering the patients’ demographic, pregnancy, and surgical data and the REEDA Scale.
The REEDA Scale was developed by Davidson (1974) to evaluate the perineal conditions. This scale measures five symptoms, such as Redness (Hyperaemia), oedema, ecchymosis, discharge, and approximation of the wound edges (Coaptation). Each symptom is scored 0 to 3, with 0 indicating “absence of the symptom” and 3 indicating “the existence of severe symptom.” The total score is interpreted as follows: 0 means “recovery,” a score of 1 - 5 indicates “moderate recovery,” a score of 6 - 10 indicates “weak recovery,” and a score of 11 - 15 indicates “no recovery” (32). In the present study, a score greater than 6 was considered to mean the occurrence of surgical wound infection.
The content validity of the REEDA Scale was confirmed by Pore by administering it to 40 doctors and nurses, and its reliability was confirmed with Cronbach's alpha of 0.9 (33). Moreover, Amani et al. assessed the reliability of the REEDA Scale through inter-rater agreement. To this end, the scale was simultaneously administered by the researcher and a subject-matter expert for 10 women on the fifth day after delivery, and Spearman's test was used to measure the inter-rater correlation. The resulting value was 0.85, confirming the reliability of the instrument (34).
The participants’ demographic, pregnancy, and surgical data were collected through interviews with the patients and by reviewing their medical files. The patients in the intervention group were treated using the care bundle for the prevention of surgical site infection. To do so, pre-surgery care was performed for the patients first. After the patient was called to the operating room, intraoperative care was provided followed by postoperative care in the recovery room and the surgical department. The SSI prevention care bundle focused on preoperative, intraoperative, and postoperative nursing interventions.
Preoperative nursing interventions involved taking a shower the day before surgery preferably with an antibacterial detergent, trimming excess hair at the surgical site with an electric clipper preferably one hour before sending the patient to the operating room, washing the surgical site with a 2% chlorhexidine disinfectant solution, dressing the surgical site with sterile gauze and prophylaxis antibiotic injection one hour before sending the patient to the operating room. These procedures were performed in the Gynecological Surgery Department. Intraoperative nursing interventions involve preparing the surgical site with a 7.5% brown povidone-iodine disinfectant solution, dyeing the surgical site with a 10% green povidone-iodine disinfectant solution, accurately controlling arterial blood oxygen saturation (SpO2) above 95% and oxygen therapy (if needed), strict control of glucose (less than 200mg/dL), and maintaining the patient's normothermia (above 36°C) during surgery and in the recovery room by using hot water bags and blankets.
Postoperative nursing interventions involve maintaining the surgical site dressing for at least 24 hours after surgery, washing and dressing the wound with sterile waterproof dressing prepared by the researcher on the second day after surgery, teaching the patient how to take care of the wound at home (daily shower, washing the wound with baby shampoo and drying it with a hair dryer or warm towel from 48 hours after the operation), full use of the prescribed oral antibiotic, proper nutrition containing protein, taking iron and vitamin A and C, using an abdominal brace (preventing the abdomen from falling on the surgical incision), teaching the symptoms of surgical wound infection, and the completion of the wound care checklist by the patient.
The patients in the control group received only the routine care of the surgical department and operating room. The surgical incision was examined through direct observation and using the REEDA Scale by the researcher 24 hours after surgery at the time of changing the dressing, each time of washing the wound, on the tenth day at the time of suture removal, and on the thirtieth day.
The patients in both groups were followed up for one month after surgery. An educational pamphlet about wound infection symptoms was given to the patients at the time of education during the discharge so that they could refer if any of the symptoms of infection occurred. From the time of discharge until 30 days after surgery, the researcher contacted the patients once a week and reminded them about how to take care of the wound and complete the checklist. The collected data were analyzed with SPSS-21 software at a significance level of less than 0.05 (P < 0.05). The data were summarized using descriptive statistics (frequency, mean, and standard deviation). First, the normality of the data was checked using the Shapiro-Wilk test, and since the data followed a normal distribution pattern, parametric tests were used for data analysis. Independent samples t-test was used to compare the quantitative variables between the two groups, and the chi-square test was used to check the independence or dependence of qualitative variables with each other. The chi-square test was also used to check the incidence of infection in the two groups.
4. Results
This study was conducted on 60 pregnant women candidates for non-emergency cesarean section admitted to Ali Ibne Abitalib Hospital in Zahedan. The patients were divided into the control and intervention groups (each with 30 patients). Parametric tests were used to analyze the data. The average age of patients in the intervention group was 28.96 years and that in the control group was 27.66 years. The independent samples t-test did not show a significant difference between the two groups in terms of age (P = 0.4).
The independent samples t-test showed no significant difference between the two groups in terms of age, body mass index, gestational age, number of pregnancies, hemoglobin, systolic and diastolic blood pressure, temperature, glucose level, SPO2 before and after surgery, and REEDA score. The chi-square test showed no significant intergroup difference in terms of education and incidence of infection. Moreover, Fisher's exact test for occupation, ethnicity, and cesarean section showed that the patients in the two intervention and control groups had no statistically significant differences in terms of demographic, pregnancy and surgical characteristics, except for the history of infection (Table 1).
| Variables and Categories | Intervention Group | Control Group | P-Value |
|---|---|---|---|
| Age | 28.96 ± 6.08 | 27.66 ± 5.9 | 0.4 b |
| BMI | 27.06 ± 2.82 | 25.83 ± 2.17 | 0.06 b |
| Gestational Age | 38.46 ± 0.62 | 38.4 ± 0.49 | 0.65 b |
| Gravida | 3.33 ± 1.72 | 3.33 ± 1.2 | 0.07 b |
| Education | 0.07 c | ||
| Illiterate | 12 (40) | 6 (20) | |
| High school | 14 (46.67) | 13 (43.33) | |
| Diploma and above | 4 (13.33) | 11 (36.67) | |
| Job | 0.39 d | ||
| Housewife | 28 (93.33) | 26 (86.67) | |
| Employed | 2 (6.67) | 4 (13.33) | |
| Nationality | 0.28 d | ||
| Baloch | 18 (60) | 21 (70) | |
| Sistani | 8 (26.67) | 6 (20) | |
| Other | 4 (13.33) | 3 (10) | |
| Cesarean section | 0.07 d | ||
| First | 4 (13.33) | 8 (26.7) | |
| Second | 8 (26.67) | 14 (46.7) | |
| Third | 4 (13.3) | 2 (6.7) | |
| Fourth and more | 14 (46.7) | 6 (20) | |
| History of Infection | 0.01 c | ||
| Yes | 6 (20) | 0 (0) | |
| No | 24 (80) | 30 (100) |
a Values are expressed as No. (%) or mean ± SD.
b Independent samples t-test.
c Chi-square test.
d Fisher's exact test.
Since the history of infection was significantly different between the two groups, this variable was included in the regression model. However, regression analysis showed that the history of infection was not a significant predictor of the incidence of infection between the two groups (P = 0.576).
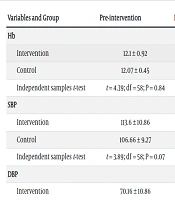
Moreover, the independent samples t-test showed no statistically significant difference in the mean REEDA score for the patients in the intervention and control groups 24 hours after surgery (P = 0.41), on the 10th day (P = 0.12), and on the 30th day after surgery (P = 0.07) (Table 2). In total, 2 patients (6.67%) in the intervention group and 5 patients (16.67%) in the control group had surgical site infection, but the chi-square test showed no significant difference between the two groups (P = 0.22) (Tables 3 and 4).
| Variables and Group | Pre-intervention | Post-intervention |
|---|---|---|
| Hb | ||
| Intervention | 12.1 ± 0.92 | 11.42 ± 0.76 |
| Control | 12.07 ± 0.45 | 11.51 ± 0.58 |
| Independent samples t-test | t = 4.39; df = 58; P = 0.84 | t = 4.48; df = 58; P = 0.76 |
| SBP | ||
| Intervention | 113.6 ± 10.86 | 100.96 ± 14.22 |
| Control | 106.66 ± 9.27 | 102.13 ± 13.55 |
| Independent samples t-test | t = 3.89; df = 58; P = 0.07 | t = 1.52; df = 58; P = 0.13 |
| DBP | ||
| Intervention | 70.16 ± 10.86 | 61.7 ± 10.24 |
| Control | 64.66 ± 11.05 | 61.36 ± 10.21 |
| Independent samples t-test | t = 3.24; df = 58; P = 0.003 | t = 1.29; df = 58; p = 0.20. |
| Temperature | ||
| Intervention | 36.73 ± 0.33 | 36.20 ± 0.43 |
| Control | 36.67 ± 0.28 | 36.26 ± 0.34 |
| Independent samples t-test | t = 5.13; df = 58; P = 0.61 | t = 5.61; df = 58; 0.42 = p |
| Glucose | ||
| Intervention | 87.86 ± 6.73 | 81.63 ± 17.18 |
| Control | 85.6 ± 11.10 | 79.63 ± 7.63 |
| Independent samples t-test | t = 1.83; df = 58; P = 0.07 | t = 3.55; df = 58; P = 0.08 |
| Spo2 | ||
| Intervention | 98.56 ± 0.89 | 97.63 ± 0.85 |
| Control | 97.33 ± 1.06 | 97.26 ± 0.78 |
| Independent samples t-test | t = 4.78; df = 58; 0.87 = p | t = 0.29; df = 58; P = 0.77 |
| Group | Second Day | Tenth Day | Thirtieth Day |
|---|---|---|---|
| Intervention | 4.6 ± 2.52 | 2.7 ± 1.3 | 0 |
| Control | 5.26 ± 3.64 | 3.07 ± 1.7 | 0.3 ± 0.08 |
| Independent samples t-test | t-test = -0.82; df = 58; P = 0.41 | t-test = 1.04; df = 58; P = 0.12 | t-test = 1.6; df = 58; P = 0.07 |
| Variables and Categories | Intervention Group | Control Group | P-Value |
|---|---|---|---|
| Infection | 0.22 a | ||
| Yes | 2 (6.67) | 5 (16.67) | |
| No | 28 (93.33) | 25 (83.33) |
a Chi-square test.
5. Discussion
The present study examined the effect of the care bundle on cesarean section wound infection and the results showed that the incidence of SSI in the patients in the intervention group who received the care bundle and the control group did not have a statistically significant difference. However, the difference in SSI was clinically important. The patients in the two groups showed no significant difference in terms of demographic and pregnancy characteristics such as age, education, ethnicity, occupation, BMI, gestational age, the reason for cesarean section, number of cesarean sections, hemoglobin/hematocrit, blood glucose and blood pressure, cesarean section, postoperative temperature, and arterial blood oxygen saturation.
Other studies (e.g. Tanner et al. (35); Jurt et al. (36); Anthony et al. (37)) reported similar findings. Anthony et al. examined the effect of the evidence-based care bundle compared to the standard method on the prevention of surgical site infection in patients undergoing colorectal surgery and showed that the bundled interventions had no effect on reducing the incidence of surgical site infection, and the hypothesis that the care bundle is effective in reducing the rate of surgical site infection in patients undergoing elective colorectal surgery was rejected, as reported in the present study (37). In addition, Jurt et al. evaluated the effectiveness of a care bundle based on multifaceted evidence including 9 intraoperative items, and found that the care bundle did not affect the incidence of surgical site infection due to medical staff’s inadequate compliance with individual measures (36).
A prospective cohort study by Tanner et al. examined the effectiveness of a care bundle in reducing surgical site infections in patients with colorectal surgery and showed that the care bundle did not reduce colorectal surgery site infection. However, it cannot be argued that the care bundles are not effective in preventing postoperative infections (35), as many studies have shown the positive effect of the surgical site infection prevention care bundle (38-44). Zajac et al. confirmed that a bundled care approach to preventing post-cesarean surgical site infection reduces surgical site infection in cesarean-section patients (44). Perhaps one reason for no significant difference in the frequency of surgical infections in the two groups was that the patients in the control group received wound care training from a gynecological surgeon and nurses at the time of discharge. Ponnampalavanar et al. also stated infection prevention preoperative and postoperative training for patients also plays an important role in reducing surgical site infections. They also showed that the cesarean section infection prevention bundle can reduce the incidence of surgical site infection after cesarean section (41).
Furthermore, Davidson et al. showed that the implementation of the SSI care bundle including preoperative, intraoperative, and postoperative care led to a significant decrease in the incidence of cesarean section SSI (38). These conflicting findings can be attributed to the methodology used in this study as the patients were divided into two groups before and after the use of the care bundle. Then, the overall prevalence of cesarean section SSIs was assessed during the study period. However, the present study was conducted in a short period and on a limited number of patients. Mahomed et al. also showed the effect of implementing an evidence-based care bundle in reducing post-cesarean surgical site infection. Interventions such as vaginal cleaning and adjusting the dose of antibiotic prophylaxis for patients based on BMI (40), which are different from care bundle interventions in the present study can account for inconsistent results reported by previous studies.
Bagga et al. suggested that the incidence of SSIs was lower in patients receiving a care bundle due to the provision of knowledge about simple measures to prevent SSI to patients and healthcare staff, including doctors. This was also attributed to increased compliance of patients with simple measures such as taking a bath before surgery, washing the scalp, and providing facilities (scissors for removing hair from the surgical site and taking a bath before surgery). They stated that prophylactic antibiotic injection by the anesthesia team in the operating room instead of nursing staff in the surgical department may contribute to reducing the burden of SSIs (42). In the present study, surgical site infection prevention training was provided to the patients in the two groups before elective cesarean section surgery by ward residents and nurses, which may account for no significant intergroup difference in terms of the incidence of surgical site infection. Moreover, the implementation of some components of the care bundle, such as evaluation of patients on the day before surgery by gynecology/obstetrics residents, training the patient to take a shower the night before surgery and shave the surgical site, prescribing antibiotic prophylaxis, probing the surgical site, receiving training on wound care, and the use of an abdominal brace at the time of discharge for the patients in both intervention and control groups can account for why no significant difference was found between the two groups. Another reason is elective cesarean section because patients undergoing this type of surgery have enough time to receive the necessary training such as proper hair removal from the surgical site and taking a shower before surgery.
The present study showed that the incidence of surgical site infection in the patients in the intervention group (recipients of the care bundle) was not significantly different compared to the control group, but this finding does not confirm the ineffectiveness of the care bundle. Moreover, some similarities between the bundled interventions conducted for the patients in the intervention group and the routine care provided to the patients in the control group can account for why no significant difference was found between the two groups. In addition, many studies have confirmed the effect of the care bundle in reducing surgical site infections. For instance, a meta-analysis revealed that evidence-based care bundles are promising interventions to reduce the risk of surgical site infection in women undergoing cesarean section because these preventive care bundles are a group of evidence-based interventions that are effective as a whole instead of individual interventions (43). Hence, the findings from the present study cannot deny the positive effect of using preventive care bundles on the incidence of surgical site infection. Accordingly, the components of care bundles should be carefully examined, and any potential issues need to be more carefully addressed in subsequent studies, especially intraoperative interventions such as maintaining the patient's normothermia during surgery and in the recovery room and the operating room.
5.1. Conclusions
The findings from the present study showed that the cesarean wound infection prevention care bundle used in this study did not affect the incidence of surgical site infections. Thus, given the effectiveness of the care bundles in some studies, these bundles should be streamlined by revising the components of the care bundle for different surgeries, especially those surgeries in which the probability of SSI occurrence is higher.