1. Background
Chronic kidney failure is an irreversible decline in kidney function that, without hemodialysis or a kidney transplant, can be fatal (1). The global prevalence of chronic kidney disease (CKD) has risen significantly, leading to higher mortality rates, complications, and reduced quality of life for patients. Currently, around 850 million people worldwide suffer from kidney disorders, with projections indicating that by 2040, CKD will be the fifth leading cause of lost years of life globally (2). In Iran, the prevalence of CKD in 2024 was reported at 18.4%, with regional variations ranging from 6.2% to 32.7% (3). Hemodialysis is a common treatment for CKD, used by over 89% of end-stage kidney disease (ESKD) patients worldwide (4). While dialysis improves patient survival, it presents significant challenges, including reduced physical capacity, impaired daily functioning, fatigue, immobility, poor sleep quality, and overall diminished well-being (5). These issues profoundly impact patients' quality of life, contributing to feelings of hopelessness, depression, reduced self-esteem, and social isolation (6). Fatigue is one of the most common and debilitating symptoms for hemodialysis patients, severely affecting their cognitive, physical, and psychological functions. Patients often experience poor concentration, persistent drowsiness, excessive daytime sleepiness, and a diminished ability to perform daily activities (7). A systematic review revealed that the prevalence of fatigue in dialysis patients ranges from 20% to 86% (8), while in Iran, chronic fatigue in hemodialysis patients is estimated to be between 60% and 97% (9). Several factors contribute to fatigue in these patients, including anemia, dialysis frequency and intensity, age, body size, malnutrition, uremia, high cholesterol, depression, and behavioral factors. Addressing fatigue in CKD patients is crucial, as managing it directly impacts health and survival outcomes (7). In addition to fatigue, treatment adherence plays a crucial role in enhancing patient health and quality of life. However, non-adherence to treatment regimens is prevalent among hemodialysis patients. A study conducted in Palestine reported adherence rates of 24% for dietary restrictions, 31% for fluid intake limits, and 52% for dialysis sessions (10). A systematic review in Iran highlighted several barriers to treatment adherence, including patient-related factors, socio-economic conditions, psychological issues, healthcare system challenges, and aspects of the disease itself. Non-adherence contributes to higher hospitalization rates and increased mortality (11). Sleep disorders are another prevalent issue among hemodialysis patients, further degrading their quality of life. A systematic review found that 68% of hemodialysis patients suffer from poor sleep quality (12), with studies in Iran estimating this prevalence at 75% (13). A study by Hosseini et al. found that 78% of hemodialysis patients experience poor sleep quality, which significantly correlates with reduced health-related quality of life. Sleep disturbances are also linked to increased hypertension, fatigue, and depression (14). Despite advancements in ESKD treatment and improved survival rates, no definitive cure currently exists. With the shift from disease-centered to patient-centered care, patients must take an active role in managing their condition (15). Self-management is a behavioral modification strategy and an ongoing, interactive process that empowers patients to control their disease and maintain optimal health (16). However, many hemodialysis patients struggle with effective self-management. For instance, Gela and Mengistu found that 57% of hemodialysis patients in Ethiopia had poor self-management (17). Similarly, Hafezieh et al. reported that self-management levels in hemodialysis patients in Yazd were moderate, with higher self-management linked to better education, knowledge, and self-efficacy (17, 18). Research on the effectiveness of self-management interventions for hemodialysis patients shows mixed results. Some studies suggest that these programs improve knowledge (19), self-care, self-efficacy (20, 21), and adherence to dietary, fluid, and medication regimens (22). However, other studies report no significant impact on serum sodium and potassium levels (23) or certain quality of life aspects (24). In Iran, limited research exists on the effectiveness of self-management interventions in improving health outcomes for hemodialysis patients (25). Additionally, there is a lack of scientific evidence regarding the impact of self-management programs on fatigue, sleep quality, and treatment adherence among hemodialysis patients in this population.
2. Objectives
This study aimed to determine the impact of a self-management program on fatigue, treatment adherence, and sleep quality in hemodialysis patients.
3. Methods
This quasi-experimental study, utilizing a pre-test-post-test design with a control group, was conducted in Zahedan teaching hospitals in 2021. The study protocol was approved by the Ethics Committee of Zahedan University of Medical Sciences (ethics code: IR.ZAUMS.Rec.1398.392). The sample size was determined based on a study by Shayani Momtaz et al. (9), using a 95% confidence interval and a test power of 95%, estimating 15 participants per group. To enhance study validity and account for potential attrition, the sample size was increased to 30 participants per group, resulting in a total of 60 patients.
The study population included all CKD patients undergoing hemodialysis who met the inclusion criteria. Participants were selected using convenience sampling and assigned non-randomly to either the intervention or control group. To prevent information contamination, hemodialysis patients at Khatam Al-Anbia Hospital were assigned to the intervention group, while those at Imam Ali Hospital were included as the control group. Inclusion criteria were having at least 18 years old, undergoing at least two dialysis sessions per week for at least six months, not having any known mental/psychological disorder, having full consciousness and listening ability, and not having participated in self-care programs within the past three months. Patients who were candidates for kidney transplantation within a month, experienced life-threatening disease, or the failure to continue participating in the study, were excluded.
The instruments used to collect the data were a demographic and clinical information questionnaire (age, gender, education level, history of underlying disease, vascular access methods, dialysis adequacy, dialysis complications, number of hemodialysis sessions per week), the Multidimensional Fatigue Inventory (MFI-20), the End-Stage Renal Disease Adherence Questionnaire (ESRD-AQ), and the Pittsburgh Sleep Quality Index (PSQI). The MFI-20, developed by Smets et al. (26), assesses fatigue across five dimensions: General fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue. It consists of 20 items rated on a five-point Likert Scale (1 = completely true to 5 = completely false). Each subscale has a score range of 4 to 20, with a total score between 20 and 100, where higher scores indicate greater fatigue. The validity and reliability of the Persian version have been confirmed in Iran (9), and in the present study, the reliability was confirmed with a Cronbach’s alpha of 0.93.
The ESRD-AQ, developed and validated by Kim et al. (27), evaluates adherence to treatment in patients with end-stage renal disease. It consists of 46 questions divided into five sections: General information (5 items), adherence to hemodialysis (14 items), medication adherence (9 items), fluid intake restriction (10 items), and dietary adherence (8 items). The total adherence score ranges from 0 to 1200, with higher scores indicating better adherence. The Persian version of this questionnaire has demonstrated acceptable validity and reliability in Iran (28). In the present study, its reliability was confirmed with a Cronbach’s alpha of 0.87.
The PSQI, developed by Buysse et al. (29), evaluates sleep quality over the past month. It consists of 18 items measuring seven components: Subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. Each component is scored from 0 to 3, with a total score ranging from 0 to 21, where a score greater than 5 indicates poor sleep quality. The Persian version of this questionnaire has demonstrated strong psychometric properties in Iran (14), and in this study, the reliability was confirmed with a Cronbach’s alpha of 0.92.
After obtaining permission from the ethics committee, patients who met the study’s enrollment criteria were selected using convenience sampling. Written informed consent was obtained from all participants before data collection. The patients in both groups completed the questionnaires as the pretest. The participants in the intervention group attended a self-management program that lasted 4 sessions in the hemodialysis unit, each lasting 30 to 45 minutes. Sessions were conducted 2 to 3 times per week, aligned with the patients' hemodialysis schedule to ensure feasibility and minimize disruption. Education was provided using a face-to-face teach-back approach, targeting both the patient and their family caregiver. The sessions were interactive, focusing on self-management behaviors and individualized needs assessment through goal setting, identifying barriers, and problem-solving strategies. The content was developed based on self-management concepts, validated by experts, and compiled into an educational booklet, which was provided to patients at the end of the intervention (Table 1). The control group received routine care without any intervention. One and three months after the completion of the intervention, a post-test was administered to both the intervention and control group.
| Session | Session Content |
|---|---|
| 1 | Factors contributing to kidney failure/introduction to dialysis treatment/assessment of the/needs assessment/significance of self-care and management |
| 2 | Review of the previous session and addressing challenges/discussing the importance of following treatment guidelines (diet, medication, fluid restrictions)/self-management strategies and common obstacles/improving self-management skills through patient involvement |
| 3 | Review of the previous session and addressing challenges/explaining the causes of poor sleep quality identifying solutions to improve sleep quality/strategies for self-management and overcoming obstacles/improving self-management skills through patient involvement |
| 4 | Review of the third session/causes and impact of fatigue in patients/strategies to reduce fatigue/self-management techniques and overcoming barriers/improving self-management skills through patient involvement/feedback and suggestions |
The collected data were analyzed using descriptive statistics, including frequency, percentage, mean, and standard deviation. The Shapiro-Wilk test was also used to evaluate the normality of the data. The chi-square test was applied to compare categorical variables, and independent t-tests assessed continuous variables. Although repeated measures ANOVA was initially considered, assumptions of sphericity and homogeneity of variances were not met. Therefore, a non-parametric rank-based approach (Brunner & Puri's F-type ANOVA) was applied for fatigue and adherence data. Analyses were performed using SPSS version 16 and R version 3.0.1 ("nparLD" package), with a significance level set at P < 0.05.
4. Results
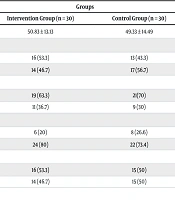
The mean age of patients in the intervention and control groups was 50.83 ± 13.13 years and 49.33 ± 14.49 years, respectively. An independent t-test showed no statistically significant difference between the groups (P = 0.67). In terms of education level, the majority of patients in both the intervention (63.3%) and control (70%) groups were either illiterate or had only primary education. The chi-square test indicated no significant statistical difference between the groups in this regard (P = 0.58). Other demographic and clinical characteristics of the participants are summarized in Table 2.
| Variables and Categories | Groups | P-Value | |
|---|---|---|---|
| Intervention (n = 30) | Control (n = 30) | ||
| Age, mean ± SD | 50.83 ± 13.13 | 49.33 ± 14.49 | 0.67 b |
| Sex | 0.43 c | ||
| Male | 16 (53.3) | 13 (43.3) | |
| Female | 14 (46.7) | 17 (56.7) | |
| Education level | 0.58 c | ||
| Illiterate/elementary | 19 (63.3) | 21(70) | |
| Diploma and higher | 11 (36.7) | 9 (30) | |
| Number of hemodialysis sessions per week | 0.54 c | ||
| Twice | 6 (20) | 8 (26.6) | |
| Three times | 24 (80) | 22 (73.4) | |
| Dialysis adequacy | 0.79 c | ||
| Favorable | 16 (53.3) | 15 (50) | |
| Unfavorable | 14 (46.7) | 15 (50) | |
a Values are expressed as No. (%) unless otherwise indicated.
bt-test.
c Chi-square test.
Regarding sleep quality, the highest scores were observed three months post-intervention, while the lowest were recorded at baseline. Statistical assumptions were tested, revealing violations in covariance matrix equality and sphericity, leading to the use of Wilks' Lambda and Greenhouse-Geisser correction. Levene’s test confirmed that error variance remained consistent across time points (P > 0.05). The independent t-test showed no baseline difference in sleep quality scores (P = 0.648). Repeated measures ANOVA indicated no significant effect of time (P = 0.118), group (P = 0.636), or their interaction (P = 0.361), meaning sleep quality did not significantly change over time or differ between groups (Tables 3 and 4).
| Source of Variation | Wilks' Lambda | F | df | Error df | P-Value | Effect Size |
|---|---|---|---|---|---|---|
| Time | 0.928 | 2.221 | 2 | 57 | 0.118 | 0.072 |
| Time × group | 0.965 | 1.036 | 2 | 57 | 0.361 | 0.035 |
| Source of Variation | Sum of Squares | df | Mean Square | F | P-Value | Effect Size |
|---|---|---|---|---|---|---|
| Group | 62.09 | 1 | 62.09 | 0.226 | 0.636 | 0.004 |
| Error | 15933.62 | 58 | 274.72 | - | - | - |
For fatigue scores, a non-parametric F-type ANOVA was used due to assumption violations. Both independent t-test and Mann-Whitney U test confirmed no baseline difference (P = 0.283). The main effects of time (P = 0.234), group (P = 0.375), and their interaction (P = 0.350) were also not significant (Table 5).
| Effect | ANOVA F Statistic | df | P-Value |
|---|---|---|---|
| Time | 1.453 | 1.761 | 0.234 |
| Group | 0.786 | 1 | 0.375 |
| Time × group | 1.027 | 1.761 | 0.350 |
Similarly, treatment adherence scores showed no baseline difference between groups (P = 0.069, confirmed by Mann-Whitney U test, P = 0.079). While time (P = 0.083) and time-group interaction (P = 0.155) were not significant, the main effect of group was significant (P = 0.013), indicating a difference in adherence between the two groups. This was confirmed by the adjusted F statistics, supporting the significance of the group effect (Table 6).
| Effect | ANOVA F Statistic | df | P-Value |
|---|---|---|---|
| Time | 2.610 | 1.69 | 0.083 |
| Group | 6.202 | 1 | 0.013 a |
| Time × group | 1.909 | 1.69 | 0.155 |
a P < 0.05 is significantly level.
5. Discussion
The findings of the present study showed that the implementation of a self-management intervention in hemodialysis patients significantly improved treatment adherence in the intervention group. However, no significant differences were observed between the intervention and control groups regarding fatigue and sleep quality after the intervention. The impact of the self-management program on sleep quality in hemodialysis patients revealed no significant difference in the overall mean sleep quality scores between the intervention and control groups. Additionally, the main effects of time, group, and their interaction were not statistically significant. Consistent with these findings, Kim reported no significant differences in sleep disturbances between hemodialysis patients receiving self-management interventions and those in the control group (20). Sleep disturbances and poor sleep quality are common among hemodialysis patients and are influenced by multiple factors, including age, underlying diseases, pruritus, and depression (12). A study by Pan et al. found that 70% of hemodialysis patients experience poor sleep quality and highlighted the role of social support and education in improving sleep quality (30). This finding contrasts with the present study, potentially due to differences in intervention components and the absence of social support assessment in our research. Furthermore, variations in geographical regions, study populations, and methods of sleep quality assessment may contribute to these discrepancies. Borzou et al. reported that face-to-face education improved specific dimensions of sleep quality in hemodialysis patients, a finding inconsistent with our results (31). Given the multifactorial nature of sleep quality, future studies should explore direct interventions such as relaxation techniques, massage therapy, pruritus management, and depression treatment. Additionally, multidisciplinary approaches addressing psychological, social, nutritional, and physical activity-related factors are recommended.
Treatment adherence scores were higher in the intervention group one month after the intervention compared to the control group; however, this difference was not sustained at the three-month follow-up. The decline in adherence over time may be attributed to factors such as an initial improvement in symptoms, decreased motivation to continue the adherence program, or a reduction in emotional support from family members (20). Yangöz et al. reported that self-management interventions positively influenced adherence to fluid intake, dietary restrictions, medication management, and serum phosphorus and potassium levels in patients with renal failure. However, only minimal differences were observed in certain aspects of treatment adherence (22). Similarly, Park and Kim found that an integrated self-management program enhanced self-efficacy and treatment adherence in hemodialysis patients (23). These findings contrast with the present study, which may be due to differences in intervention content, delivery methods (e.g., face-to-face vs. distance learning), and demographic or cultural factors. Notably, previous studies have emphasized the role of self-efficacy in improving self-management, an aspect not evaluated in the present research.
The findings regarding the impact of the self-management program on fatigue in hemodialysis patients indicated no significant differences in mean fatigue scores between the intervention and control groups at one- and three-months post-intervention. Fatigue in hemodialysis patients is a multifaceted symptom influenced by biological, behavioral, psychological, and social factors. It is closely linked to sleep disturbances, social and family functioning, physical activity levels, underlying diseases, serum creatinine levels, and dialysis adequacy (7). High levels of fatigue are often observed in unemployed individuals and those with limited social support, whereas strong family and social networks are associated with lower fatigue levels (12). The present study did not assess patients' social and family support, which should be considered in future research. Additionally, many participants in this study had diabetes and hypertension, and nearly half were unemployed and physically inactive, which may have contributed to persistent fatigue levels. Borzou et al. demonstrated that health education effectively reduced fatigue in hemodialysis patients, a finding inconsistent with our results (31). A potential explanation for this inconsistency is the direct connection between fatigue and sleep hygiene. Due to the complex nature of fatigue, additional research is needed, and the results should be interpreted with caution.
This study has several limitations. The generalizability of the findings is restricted due to the limited sample size and the fact that the study was conducted in only two hospitals. Additionally, data collection was based on self-reported measures, which may have introduced response bias. Despite these limitations, a strength of the study was the design and implementation of a self-management program tailored to the individual needs of patients.
5.1. Conclusions
The findings of this study indicate that the self-management program did not significantly improve sleep quality or reduce fatigue in patients with CKD. However, in the first month following the intervention, treatment adherence was higher in the intervention group compared to the control group. Given the complex and multifactorial nature of sleep quality and fatigue, future interventions should incorporate psychological, social, nutritional, and physical activity components to enhance their effectiveness. Further research is also needed to identify the barriers and predictors affecting intervention outcomes. Considering the observed short-term improvement in treatment adherence, integrating self-management programs into dialysis care — led by nurses — may be beneficial. Additionally, longitudinal studies should explore the factors contributing to non-adherence over time to develop more effective strategies for sustained patient engagement.