1. Background
Head and neck cancer (HNC) is a significant global health concern, ranking as the third most prevalent cancer worldwide, with 1,464,550 new cases and 487,993 deaths reported in global cancer statistics 2020 (1). This cancer includes malignancies originating from the skin, nasal cavity, paranasal sinuses, oral cavity, salivary glands, pharynx, and larynx, affecting critical functions such as speech, swallowing, and eating, which severely impact patients' quality of life (2, 3).
The incidence of HNC is rising (4), particularly in developing countries such as Iran, where between 2003 and 2009, a total of 25,952 cases were recorded. The age-standardized incidence rate increased from 4.8 per 100,000 in 2003 to 8.5 in 2008 and 7.4 in 2009, reflecting a worrying upward trend (5). Given its increasing prevalence and debilitating effects, HNC requires urgent attention to improve prevention, management, and treatment strategies. Treatment modalities for HNC include surgery, chemotherapy, and radiotherapy, each aiming to eliminate the disease and improve survival. However, these treatments often lead to severe complications, including xerostomia (dry mouth), mucositis, pain, dysphagia, and alterations in taste (6-8).
Among these treatments, radiotherapy is the most widely used but also causes the most severe and long-lasting complications. It permanently damages the salivary glands, leading to a significant reduction in saliva production and alterations in its composition. The irreversible loss of salivary function contributes to various oral health problems, such as an increased risk of dental caries due to the loss of saliva’s natural protective properties, difficulty in maintaining oral hygiene, increasing the risk of periodontal disease and infections, and changes in the oral microbiome, as radiotherapy reduces immunoglobulin A secretion, disrupting bacterial balance and accelerating demineralization and tooth decay (9, 10). These complications not only compromise oral health but also significantly impact patients' overall well-being, making basic functions such as chewing, swallowing, and speaking difficult. Consequently, many patients experience nutritional deficiencies and a decline in their quality of life (11).
Given the serious impact of radiotherapy on oral health, early intervention and preventive strategies are crucial. Despite numerous studies (12-14) addressing the oral complications of radiotherapy, there is still a lack of comprehensive training programs focused on managing these issues effectively. Providing structured training programs on oral hygiene practices, nutritional support, and symptom management can significantly improve patients' quality of life during and after treatment. Given the increasing age-standardized incidence rate of HNC in Iran, developing effective educational interventions and integrating preventive oral health strategies into cancer treatment protocols is essential to minimize treatment-related complications.
2. Objectives
Therefore, this study was conducted to evaluate the effect of a care training intervention on oral health and radiation-induced xerostomia in patients with HNC.
3. Methods
This quasi-experimental study was conducted on patients with HNC undergoing chemotherapy and radiotherapy at Khatam al-Anbia and Ali ibn Abi Taleb hospitals, affiliated with Zahedan University of Medical Sciences, from December 2022 until January 2023. The sample size was determined based on a previous study using the following formula with a 95% confidence interval and 80% statistical test power, resulting in a required sample of 27 participants per group (15). To account for potential attrition and ensure an adequate sample size, this number was increased to 30 participants per group, totaling 60 individuals.
S1 = 21.53, S2 = 22.04,
Following the research project's approval by the Zahedan University ethics committee and obtaining the necessary institutional permissions, participants were recruited from chemotherapy departments using a convenience sampling method. Eligible participants were randomly assigned to either the intervention or control groups using a simple randomization technique. To implement this, colored cards (blue for the intervention group and red for the control group) were prepared, shuffled, and allocated to participants sequentially. Participants were included in the study if they were between 20 and 60 years old at the beginning of treatment, had no evidence of metastasis, possessed basic literacy skills, did not have oral infections at study entry, were not taking medications affecting salivary function, and were starting chemotherapy from the first session. Exclusion criteria included voluntary withdrawal from the study, disease progression or metastasis during the study period, patient death, or absence from at least one of the training sessions.
Data collection was carried out using several validated instruments. A demographic and clinical information form was used to gather data on variables such as age, sex, education level, employment status, primary caregiver, place of residence, history of comorbid conditions (e.g., diabetes), history of substance use, neutrophil-to-lymphocyte ratio (NLR), and Body Mass Index (BMI). The FOX Xerostomia Questionnaire, a 10-item validated tool, was used to assess subjective xerostomia, with a score of at least four positive responses indicating the presence of xerostomia. The validity and reliability of this questionnaire have been confirmed in previous Iranian studies (16), and in this study, its reliability was confirmed with a Cronbach’s alpha of 0.85. Objective xerostomia was assessed using the RTOG/EORTC Xerostomia Grading Scale, a four-level classification system widely used in clinical research (Table 1). The reliability of this instrument was verified with a Cronbach’s alpha of 0.84. Additionally, the Decayed, Missing, and Filled Teeth (DMFT) Index was employed to quantify dental health, with a lower score indicating better oral health status. The reliability of this index in the present study was confirmed with a Cronbach’s alpha of 0.83.
| Variable | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Objective xerostomia | Normal moisture | Low salvia | No moisture/sticky salvia | No moisture |
Before the intervention, participants in both groups were informed about the study’s objectives, and written informed consent was obtained. Baseline dental health status was assessed by a dental student using the DMFT Index, and all participants completed the demographic and clinical information form, FOX Xerostomia Questionnaire, and RTOG/EORTC Xerostomia Grading Scale. The intervention group received three individual training sessions focusing on cancer treatment-related complications and professional oral care, as detailed in Table 2, conducted by a nursing student. Each session lasted between 45 and 60 minutes and was conducted weekly before the start of radiotherapy. In the final session, an educational pamphlet summarizing the key instructions was provided to the participants. To ensure adherence to the training program, the nursing student conducted weekly follow-up visits to the hospital, monitoring compliance and addressing patient concerns for 7 consecutive weeks of radiotherapy and one week after completion of radiotherapy.
| Sessions | Content |
|---|---|
| 1 | Establishing rapport with the patients and motivating them to actively participate in managing and preventing radiotherapy side effects. Providing an overview of the intervention, including its objectives, the number of sessions, and the implementation process. Educating patients on radiation-induced complications, emphasizing the importance of proper care for cancer patients, particularly those undergoing radiotherapy. |
| 2 | Educating patients on the side effects of cancer treatments, with a focus on radiotherapy complications such as xerostomia, dermatitis, and mucositis. Providing comprehensive guidance on oral and dental care in eight key areas, including xerostomia, mucositis, dental caries, tooth pain and sensitivity, halitosis, osteonecrosis, and nutrition. Teaching strategies for maintaining optimal oral and dental health. Encouraging patient engagement by facilitating a question-and-answer session to assess their understanding and address concerns. |
| 3 | Highlighting the importance of addressing dental decay before initiating radiotherapy, including recommendations for restoration or extraction of decayed teeth. Reviewing and reinforcing the instructions provided in previous sessions. Evaluating patients’ understanding and implementation of oral and dental care guidelines. Demonstrating proper flossing and tooth brushing techniques using a dental model, followed by hands-on practice by the patients under the researcher’s supervision. Summarizing key instructions and providing patients with contact information for follow-up and additional support. |
Patients in the control group received only routine hospital-based patient education. However, to adhere to ethical considerations, they were provided with an educational booklet at the end of the study. At the end of the radiotherapy treatment, the DMFT Index was reassessed for both groups by a dental student, and participants once again completed the FOX Xerostomia Questionnaire and the RTOG/EORTC grading checklist. To minimize bias, the dental examiner assessing the patients' oral health was blinded to the group allocations.
Data were analyzed using SPSS version 26. Descriptive statistics, including mean, standard deviation, frequency, and percentage, were used to summarize demographic and clinical characteristics. The normality of the data was assessed using the Shapiro-Wilk test. Inferential statistical analyses included the independent-samples t-test, Fisher’s exact test, and chi-square test. A P-value of less than 0.05 was considered statistically significant.
4. Results
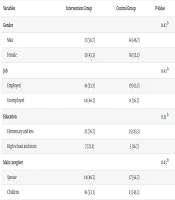
During the course of the study, three participants were excluded and replaced with eligible individuals. A total of 60 participants were enrolled, consisting of 31 males (51.6%) and 29 females (48.4%), with an age range of 29 to 58 years. The majority of participants reported no history of substance abuse (66.6%) or underlying medical conditions (76.6%). Demographic and clinical characteristics of the participants are presented in Table 3.
| Variables | Intervention Group | Control Group | P-Value |
|---|---|---|---|
| Gender | 0.43 b | ||
| Man | 17 (56.7) | 14 (46.7) | |
| Female | 13 (43.3) | 16 (53.3) | |
| Job | 0.43 b | ||
| Employed | 16 (53.3) | 19 (63.3) | |
| Unemployed | 14 (46.7) | 11 (36.7) | |
| Education | 0.51 b | ||
| Elementary and less | 23 (76.7) | 25 (83.3) | |
| High school and more | 7 (23.3) | 5 (16.7) | |
| Main caregiver | 0.43 b | ||
| Spouse | 14 (46.7) | 17 (56.7) | |
| Children | 16 (53.3) | 13 (43.3) | |
| Location | 0.43 b | ||
| City | 16 (53.3) | 19 (63.3) | |
| Village | 14 (46.7) | 11 (36.7) | |
| History of addiction | 0.27 b | ||
| Has it | 12 (40) | 8 (26.7) | |
| Does not have | 18 (60) | 22 (73.3) | |
| Age | 46.06 ± 7.75 | 45.43 ± 7.84 | 0.75 c |
| BMI | 21.08 ± 3.47 | 21.15 ± 4.80 | 0.94 c |
Abbreviation: BMI, Body Mass Index.
a Values are expressed as mean ± standard deviation or No. (%).
b Chi-square test.
c Independent t-test.
Comparison of the mean DMFT Index and its components between the two groups revealed no statistically significant differences before and after the intervention (Table 4).
| Group Variables | Pre-intervention | P-Value b | Post-intervention | P-Value b | ||
|---|---|---|---|---|---|---|
| Control Group | Intervention Group | Control Group | Intervention Group | |||
| Decayed teeth | 6.13 ± 1.77 | 6.26 ± 2.37 | 0.80 | 3.63 ± 1.88 | 3.16 ± 1.72 | 0.32 |
| Missing teeth | 6.90 ± 7.24 | 9.03 ± 7.63 | 0.27 | 7.53 ± 7.33 | 10.96 ± 7.78 | 0.21 |
| Filled teeth (filling) | 2.10 ± 2.02 | 1.23 ± 1.43 | 0.06 | 2.86 ± 21.97 | 2.53 ± 1.35 | 0.45 |
| DMFT | 15.13 ± 7.07 | 16.53 ± 7.56 | 0.46 | 15.13 ± 7.07 | 16.53 ± 7.56 | 0.46 |
Abbreviation: DMFT, decayed, missing, and filled teeth.
a Values are expressed as mean ± standard deviation.
b Independent t-test.
Regarding subjective xerostomia, none of the patients in either group reported dry mouth before the intervention. Following the educational-care intervention, 96.7% of the intervention group and 100% of the control group reported experiencing dry mouth. Fisher’s exact test indicated no significant difference between the groups (P = 1.00). Objective xerostomia levels did not differ significantly between groups either before (P = 0.317) or after the intervention (P = 0.15). While most participants initially had normal salivary moisture, a reduction in this category and a shift toward lower moisture levels were observed post-intervention in both groups. Detailed distribution across xerostomia grades is presented in Table 5.
| Variables | Pre-intervention | P-Value b | Post-intervention | P-Value c | ||
|---|---|---|---|---|---|---|
| Intervention Group | Control Group | Intervention Group | Control Group | |||
| Degree of dry mouth | 0.317 | 0.15 | ||||
| Normal moisture (grade 1) | 23 (76.6) | 26 (86.7) | 0 (0) | 0 (0) | ||
| Low saliva (grade 2) | 7 (23.3) | 4 (13.3) | 17 (56.7) | 10 (33.3) | ||
| Lack of moisture, sticky saliva (grade 3) | 0 (0%) | 0 (0%) | 12 (40) | 17 (56.7) | ||
| Lack of moisture (grade 4) | 0 (0%) | 0 (0%) | 1 (3.3) | 3 (10) | ||
| Total | 30 (100) | 30 (100) | 30 (100) | 30 (100) | ||
a Values are expressed as No. (%).
b Chi-square test.
c Fisher’s exact test.
5. Discussion
Although the intervention group exhibited a slight improvement in dental and oral health status, reflected by a lower DMFT Index compared to the control group, this difference was not statistically significant. Several factors may explain this finding. The relatively short duration of the intervention may have limited the opportunity to observe meaningful changes in the DMFT Index, which reflects cumulative oral health over an extended period. Furthermore, while the intervention may have had some positive effects, these were possibly insufficiently robust or consistent to reach statistical significance within the study’s sample size. Individual-level factors, such as oral hygiene practices, access to dental care, and baseline health literacy, may have played a more pivotal role in determining outcomes than the intervention itself. Although demographic variables were balanced between groups, behavioral variations may have influenced the results. Despite the lack of statistical significance, the observed trend toward improved oral health in the intervention group suggests potential clinical relevance, particularly regarding preventive oral care.
In addition, the care training intervention did not demonstrate a statistically significant effect on xerostomia, neither in subjective perceptions nor in objective measures of salivary flow. This suggests that although the intervention may have contributed to patient education and awareness, it was insufficient to bring about measurable improvements in either salivary flow or patients' perceived dryness of the mouth. In patients with head and neck cancer, salivary gland function is often severely compromised due to radiotherapy, which is a major and often irreversible cause of xerostomia (17). The lack of improvement in objective xerostomia in this study may be attributed to the structural damage to salivary glands that is not easily reversed through non-pharmacological approaches. Furthermore, the absence of a significant difference in subjective xerostomia may reflect the persistent perception of dry mouth among patients despite behavioral recommendations. In chronic cases, strategies such as increased water intake, sugar-free gum, or avoiding irritants may not be sufficient to alleviate symptoms perceived by the patient. It is also important to note that subjective and objective xerostomia are not always correlated (18, 19). Previous studies have shown that patients may report dry mouth even when salivary secretion is within normal limits, and conversely, some individuals with reduced salivary flow may not report noticeable dryness (19, 20). Therefore, combined interventions — including behavioral, pharmacological, and supportive strategies — may be more effective in managing xerostomia, especially in high-risk populations. Future studies should consider more comprehensive intervention models, potentially integrating salivary stimulants such as pilocarpine and extending follow-up periods to assess long-term effects on both subjective symptoms and physiological outcomes.
5.1. Conclusions
In summary, while the educational care intervention showed a non-significant trend toward improved dental health, it did not significantly impact xerostomia in patients undergoing radiotherapy for head and neck cancer. The findings highlight the complexity of managing oral complications in this population and suggest that more intensive, multifaceted interventions with longer follow-up and larger sample sizes are needed. Integrating behavioral, pharmacological, and supportive strategies may offer a more effective approach to improving both oral health outcomes and patient quality of life.
5.2. Strengths
The study’s strengths include the clinical evaluation of oral health and xerostomia by a qualified dentist, which enhanced data validity and reliability. The multidisciplinary approach, involving both nursing staff and dental professionals, facilitated a more holistic care model reflective of real-world clinical practice. Targeting a high-risk population of head and neck cancer patients further increased the study’s relevance. The educational intervention was based on evidence-based guidelines, enhancing its practical applicability.
5.3. Limitations
However, limitations such as the short intervention period, lack of direct measurement of patient adherence, small sample size, and single-center design restrict the generalizability and power of the findings.