1. Background
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic disease of the bladder along with pain and frequent and urgent urination. Chronic inflammation of the bladder is accompanied by the infiltration of macrophages, lymphocytes, mast cells and plasma cells to the bladder wall, which leads to irreversible changes in its tissues (1-3). The nature of IC/BPS is still not fully understood. Autoimmune, allergic, infectious, neurological, and vascular diseases, as well as increased infiltration of mast cells to the bladder wall, damage to mucin protective layer and the exposure of the bladder wall to the toxic substances found in the urine are among etiological factors. A variety of symptoms and differences in the susceptibility of patients to the treatment reveal the multi-etiological nature of the disease (4, 5).
Different degrees of severity of inflammation are detected in the biopsy specimens of the bladder; however, cellular mechanisms of inflammation in IC/BPS and the processes leading to tissue damage and fibrosis are not yet clear (6, 7). Among many suggested causes, mast cell activation is noteworthy (8). There is an increase in the number of mast cells and their infiltration to the bladder wall (8). Various models of cystitis in rodents showed an increased number of mast cells and their activation (9, 10). However, owing to the limited number of published studies in this regard, currently available evidence remains limited. Although most researchers have declared certain results, emphasize the complexity of the disease and the need for further research. Because of the complexity of the pathophysiology of IC/BPS, a number of animal models are used to better understand the underlying mechanisms of this disease. Modeling is often created by the intravesical instillation of protamine sulfate, the introduction of cyclophosphamide, etc. (11, 12). More than two decades, animal models with autoimmune inflammation of the bladder have been actively used in IC/BPS research (13, 14). Animal model research has shown that mast cells are responsible for bladder inflammation and pain in various models (13). However, despite this research, the role of mast cells associated with cystitis has not been identified clearly.
The data of histopathology of the bladder revealed the role of cell-mediated immunity in IC/BPS, which does not exclude the possibility that autoimmune inflammation is probably a component of pathophysiology in patients with this disease (7). Although mast cells are characteristic features of this disease, there is no consensus on their specificity and diagnostic significance. Transmural fibrosis can develop in the terminal stage of the disease, which leads to wrinkling of the bladder. The main task of a biopsy is not exactly the diagnosis of this disease, but it helps the detection of carcinoma in situ, which can mask interstitial cystitis.
2. Objectives
The aim of this study was to determine the leukocyte and mast cell infiltration to the bladder tissue in various experimental models of interstitial cystitis/bladder pain syndrome (IC/BPS).
3. Methods
Interstitial cystitis/bladder pain syndrome (IC/BPS) modeling was performed on white New Zealand female rabbits weighing 1500 - 2000 g by different methods. Animal maintenance and conducting experimental studies were based on the Rules for the Care and Use of Laboratory Animals (NIH Guide for the Care and Use of Laboratory Animals) (15). All experimental animals were divided into 4 groups (Figure 1).
The animals were exposed to hydrochloric acid (HCl), urine, 0.9% physiological saline (NaCl). In the animals of group 1, the IC/BPS was stimulated by intravesical instillation of HCl (0.2 mL of 0.5% HCl). In the animals of group 2, the IC/BPS model was created on the basis of one of the etiological theories of IC according to which uric toxicity leads to damage to the glycosaminoglycan layer (16). So suprapubic section was done and after which a urine sample was taken from the bladder with a syringe with a 30-gauge needle in a volume of 0.5 cm3 was inserted under the mucous layer of the bladder. In the animals of group 3, 10 mL of 0.9% NaCl injected into the bladder wall. The fourth group consisted of the intact animals.
To evaluate different cell content in the bladder tissues, animals were killed with pentobarbital at a dose of 200 mg/kg after 14 days. After that, they were dissected using a midline transabdominal incision and a cystectomy was performed. During the course of the experiment urine bladder extracted from the animal firstly was fixed in a 10% neutral formalin buffer solution for twenty-four hours. The next day, the sample was subjected to microscopic examination. In order to simultaneously study all layers of the bladder wall parallel sections 0.3 - 0.4 сm thick were made. The obtained samples were again stored in a 10% neutral formalin buffer solution for twenty-four hours. In subsequent days, biopsy samples were stored in various concentrations of alcohol (75%, 85%, 95%, and 99.9%) for dehydration. Subsequently, the samples were soaked in xylene solution and paraffinized. Following the follow-up process, the samples were molded and histological blocks were prepared. Sections of 3 - 5 µm were made from the obtained blocks using a microtome (Leica RM 2125 RTS, Germany) and were stained with standard May-Grünwald Giemsa staining (Merck, Germany) and hematoxylin-eosin stains for detecting mast cells (17). Prepared sections were examined by a light microscope (Leica DM 750, Germany). All changes observed during microscopic examination were recorded with a microscope attached camera (Leica ICC 50, Germany).
Microscopic examination of the bladder wall was performed with high magnification in 10 consecutive fields of view with intensive accumulation of neutrophils, lymphocytes, eosinophils, and mast cells. Each section was divided into 10 subsections; mast cell infiltration was assessed in each of these sections using the following scale: 0: no extravascular leukocytes and mast cells; 1: less than 20 leukocytes and mast cells; 2: 20 - 45 leukocytes and mast cells; 3: more than 45 leukocytes and mast cells. The points of all 10 subsections were added up, divided by 30 (the maximum possible score) and multiplied by 100. The leukocyte and mast cell scores for each bladder were the average of 3 examined sections. White blood cell and mast cell counts were performed at optical magnification ×200 (18, 19). Statistical calculations were performed with “Statistical Package for Social Sciences”, version 15.0 (SPSS 15.0). The mean and standard errors of these indicators were calculated for each group of cells.
4. Results
The study of the level of leukocyte types showed that the largest number of leukocytes was determined in the tissues of the bladder in the animals of group 1 (Table 1).
| Groups | Cell Types | |||
|---|---|---|---|---|
| Neutrophils | Lymphocytes | Eosinophils | Mast Cells | |
| 1 (n = 8) | 89.375 ± 14.927a [68; 112] | 48.375 ± 7.614a. b [36; 59] | 2.125 ± 3.226a [0; 8] | 1.125 ± 1.642 [0; 4] |
| 2 (n = 10) | 0.866 ± 1.884 [0; 6] | 29.866 ± 10.183a. b [12; 49] | 0.333 ± 0.899 [0; 3] | 14.200 ± 5.796a [3; 26] |
| 3 (n = 7) | 1.428 ± 2.699 [0; 7] | 2.285 ± 3.728b [0; 10] | 0.142 ± 0.377b [0; 1] | 0 |
| 4 (n = 7) | 0 | 0.375 ± 1.060 [0; 3] | 0.375 ± 0.744 [0; 2] | 0 |
aStatistical significance between groups (P < 0.05 - 0.001)
bStatistical significance with group 4 (control group)
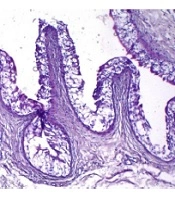
The average of all types of white blood cells in group 1 was significantly higher than the groups 2, 3, and 4 (P < 0.001). Mast cells were determined only in groups 1 and 2, and their number in the group 2 was significantly higher (P < 0.001) than group 1. Histological analysis revealed mixed lymphocyte and neutrophil infiltration against the background of extensive necrosis in the bladder wall of animals after injection of a 0.5% HCl solution (group 1) (Figure 2).
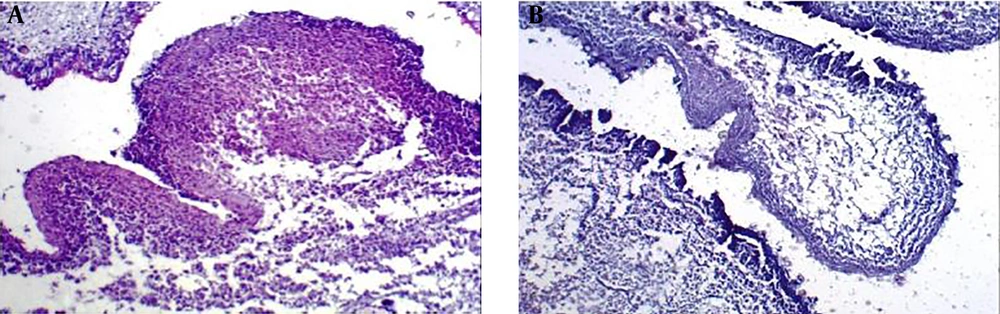
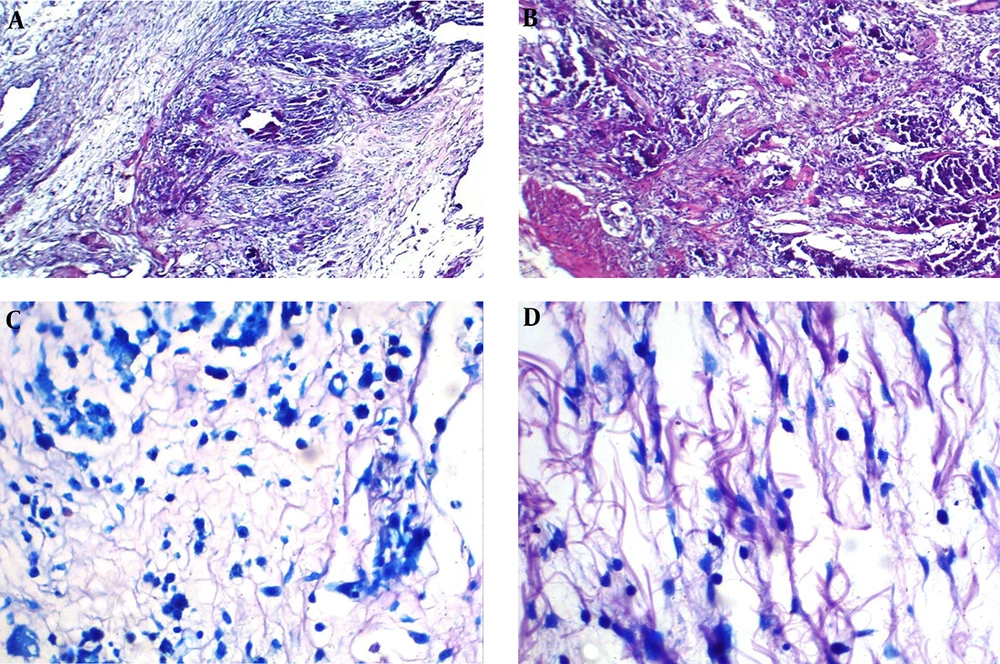
After creating a toxic model of IC/BPS, micropreparations of the bladder wall in animals of group 2 showed developing necrosis and mixed inflammatory infiltration (Figure 3A), giant necrotic multinucleated histiocytes and mixed inflammatory infiltration (Figure 3B), and mast cells (Figure 3B and C). In micropreparations of IC/BPS model obtained from the animal’s bladder after injection of 0.9% NaCl (group 3) tissue edema and single, scattered lymphocytes were noted (Figure 4).
The histological preparation of the bladder after creating a model of IC/BPS by introducing urine taken from the animal’s bladder into the wall of the bladder. (A) Developing necrosis and mixed inflammatory infiltration (×100 magnification, hematoxylin-eosin staining). (B) Developing necrosis, giant multinucleated histiocytes and mixed inflammatory infiltration (×100 magnification, hematoxylin-eosin staining). (C) Mast cell infiltration in the bladder wall (×400 magnification, May-Grünwald-Giemsa staining). (D) Mast cells infiltration in the bladder wall of experimental animal (×400 magnification, May-Grünwald-Giemsa staining).
Among intact animals (group 4) histological analysis did not reveal any abnormalities in the tissues of the bladder wall, the wall of the bladder was without any features (Figure 5). Thus, as can be seen, extensive necrosis and mixed inflammatory infiltration in the bladder wall of group 1 animals were associated with a large number of leukocytes. The histological pattern observed in micropreparations of the group 2 animals was also associated with a large number of mast cells in the bladder wall. Tissue edema was evident in histological preparations of the group 3, probably as a response to the injection, but there was no inflammatory infiltration, only single elements of inflammation were observed, which was associated with the calculation of cellular elements.
5. Discussion
In this study, we determined the infiltration of leukocytes (neutrophils, lymphocytes, eosinophils) and mast cells in the bladder tissue of animals with various options for IC/BPS modeling. Here, mast cell infiltration was detected in a chemical model (with HCl) and a model with urinary toxicity (injection of urine into the wall of the bladder). At the same time, mast cell activity in animals with a urinary toxicity model was significantly higher than the group 1 (P < 0.001).
It should be noted that mast cells are currently recognized as regulatory and effector cells of both innate and adaptive immunity (7). Their broad functions depend on their ability to respond to a wide variety of stimuli and secrete biologically active products with pro-inflammatory, anti-inflammatory and/or immunosuppressive properties. Mast cells play an important role in allergic inflammation mediated by immunoglobulins. As innate immune defenders mast cells recognize microbial agents (bacterial, viral, parasitic and fungal) and endogenous factors resulting from cell damage (7). This probably explains the absence of mast cell infiltration in animal histological sections of the groups 3 and 4 during the examination. At the same time, the statistically significant activity of mast cells in the group 2 may indicate that with this modeling option, damage to the bladder uroepithelium is more severe and mast cells react to the damage by high infiltration. The versatility of mast cells implies that they can be activated via various mechanisms. Mast cells are believed to play an important role in IC/BPS, since an increase in the number of mast cells is observed in the bladder wall of many cases (7). Animal studies have shown that mast cells play a key role in the development of bladder inflammation and pain observed in IC/BPS (13, 20).
According to our data mast cells in the bladder tissue are significantly activated under the influence of urinary toxicity. This is likely owing to the fact that the main function of the genitourinary system is to release a large number of toxic substances from the body. The bladder plays an important role by reserving, holding and secreting urine. The final metabolic products and toxins are excreted along with urine from the body. Additional consumption of these exogenous substances can lead to impaired functioning of the bladder (21, 22). Violation of the barrier function of the urothelial layer allows toxic substances from the urine to pass into the tissues (23, 24). This is exactly what we observed with the IC/BPS variant, when urine from the animal’s bladder injected into the bladder wall.
In our study, experimental groups with the injection of a 0.5% HCl solution (group 1) and the injection of urine taken from the animal’s bladder into the bladder wall (group 2) showed a high level of expression of inflammatory elements, increased lymphocyte, and neutrophil infiltration. Meanwhile, histological examination in the second variant showed the presence of mast cells infiltrates in the bladder wall, which in turn caused inflammation, as established by developing necrosis in the bladder wall of the group 2 animals. Mast cells have been found to make an important contribution to the development of the inflammatory response. When activated they release vasoactive and inflammatory mediators, causing inflammation and increasing the excitability of neuronal endings (25, 26).
5.1. Conclusions
This study demonstrates a statistically significant increase of inflammatory cells in the bladder mucosa of animals with IC/BPS simulated by injecting of 0.5% HCl solution into the bladder wall and mast cells when modeling IC/BPS by injecting urine taken from the bladder into the bladder wall. Histological analysis revealed the association of high inflammatory cell infiltration in the chemical variant of the IC/BPS model and mast cell infiltration with damage to the bladder integrity by urinary toxicity.