1. Context
Kidney transplantation (KT) is the most promising modality of treatment to improve the survival and quality of life of patients with irreversible kidney failure. However, the organ shortage limits its widespread use and clinical application. In recent decades, the utilization of the marginal and expanded criteria organs, including donation after circulatory death, has been employed to increase the organ pool (1, 2). Organs from cardiac death donors are associated with long mean ischemia time starting from the circulatory arrest of the donor to the end of the cold preservation phase before transplantation (3). The ischemia-related complications after deceased and living donations are also controversial with lower incidence rates.
The ischemia-reperfusion injury (IRI) is unavoidable tissue damage that occurs when the blood supply returns to the kidney after a period of anoxia or hypoxia. Reduced metabolic supply during prolonged blood flow disturbance causes severe capillary damage. Subsequent reperfusion not only does not restore the normal condition, but also increases the damage by activating the innate immune system and the programmed cell death process (4, 5).
Ischemia-reperfusion injury is a major cause of acute kidney injury that affects short- and long-term outcomes (6, 7). It has been demonstrated that delayed graft function (DGF) is a manifestation of IRI that is clinically defined as the “need for dialysis in the first week after transplant, once hyperacute rejection, vascular, and urinary tract complications are ruled out” (8). About 35% of first-graft patients and 47% of regrafted patients experience DGF (9). Delayed graft function occurs as a result of the activation of the innate immune response. Factors that have been found to increase the risk of DGF include donor factors (including older age, cardiac or brain death, and abnormal biopsy findings), recipient factors (including male gender, body mass index of greater than 30, African-American ethnicity, and high panel reactive antibodies), and inappropriate preservation methods.
The exact underlying cellular mechanism of IRI is not completely understood, but dysregulation of energy metabolism, alteration of cellular mitochondria and membranes, and different forms of cell death (e.g. apoptosis, necrosis, or necroptosis) have been observed in in-vivo studies. Since 1985, numerous trials have been performed in small and large animal models (e.g. rat, pig, and monkey) to test novel pharmaceutical agents administrated either intravenously or as an additive to the preservation solution. Previous studies have proven that insights gleaned from animal models can rarely be extrapolated to the human context (10). Clinical trials have been performed in human settings concentrating on different strategies including anti-inflammatory, anti-oxidant, and innate inhibition therapies. The current study abstracts published knowledge from human clinical trials and discusses the effectiveness of the proposed preventive hypotheses.
2. Evidence Acquisition
This study was conducted following the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines (11). Moreover, a priori registration of the protocol was done in PROSPERO, an international prospective register of systematic reviews (registration number: CRD42019132985) (12).
2.1. Data Sources and Search Design
A comprehensive literature search was performed in MEDLINE (via PubMed), EMBASE (via Scopus), and Science Citation Index Expanded (via Web of Science) from inception until January 1, 2019. The search strategy was developed using a Boolean combination of the subject headings or keywords associated with “Kidney”, “Transplantation”, “Reperfusion”, “Injury”, “Ischemia”, and “Prevention”. Searches were limited to human subjects and English language articles. The search terms were re-run on May 30, 2019, to capture potentially eligible studies published after the initial search.
2.2. Eligibility Criteria and Study Selection
In this review, we included the original publications of Randomized Controlled trials (RCTs) investigating the effect of an IRI prevention strategy (i.e. pharmaceutical, surgical, organ preservation, etc.) compared with a control group in a cohort of patients undergoing kidney transplantation. All studies including adult or pediatric kidney recipients from either living or deceased donors were included. Following exclusion criteria were applied to exclude irrelevant studies: meta-analyses, review articles, case reports, conference papers, abstracts, editorial letters, and in-vivo/in-vitro studies. The search results were entered in reference manager software. After deduplication, two researchers (FT and MT) independently assessed the eligibility of the citations by title and abstract. The full-texts of the relevant studies were then evaluated to be included in the final synthesis. In case of disagreement, the final decision was made after consultation with the third reviewer (SE). Once the reviewers encountered duplicate studies on an overlapping study population, the study with the larger sample size was included. The reference list of the included trials and the “Similar Articles” feature in PubMed were also used to identify additional studies.
2.3. Data Extraction and Management
One reviewer (with clinical research training) extracted the following data items using a standardized form: publication year, country or region, the number of patients in each study arm, patients’ demographics separated for the intervention and control groups (i.e. age and gender distribution), and preventive agent or technique.
The incidence rate of the DGF was considered the primary outcome. Secondary outcomes were serum creatinine, creatinine clearance, urine output, estimated glomerular filtration rate (eGFR), and graft/patient survival rates. Once statistical analyses showed significant differences for at least one outcome in favor of the intervention group, the effectiveness variable was considered as “yes”. The second reviewer checked for the validity of the data abstraction.
2.4. Risk of bias and Quality Assessment
Two investigators (FT and MT) independently assessed the methodological risk of bias using the Cochrane risk of bias tool (13). This tool categorizes the risk of bias as low, high, or unclear across the following domains: random sequence generation (selection bias), allocation concealment (selection bias), selective reporting (reporting bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and other sources of bias (e.g. fraudulent results, funding issues, etc.). We then used the thresholds defined by the Agency for Healthcare Research and Quality (AHRQ) to categorize the quality of studies as good (low risk of bias for all domains), fair (high risk of bias for one domain or unclear description for two domains), or poor (high or unclear risk of bias in two or more domains) (14). Conflicts on risk assessment were resolved by the third investigator (SE).
2.5. Data Synthesis and Statistical Analyses
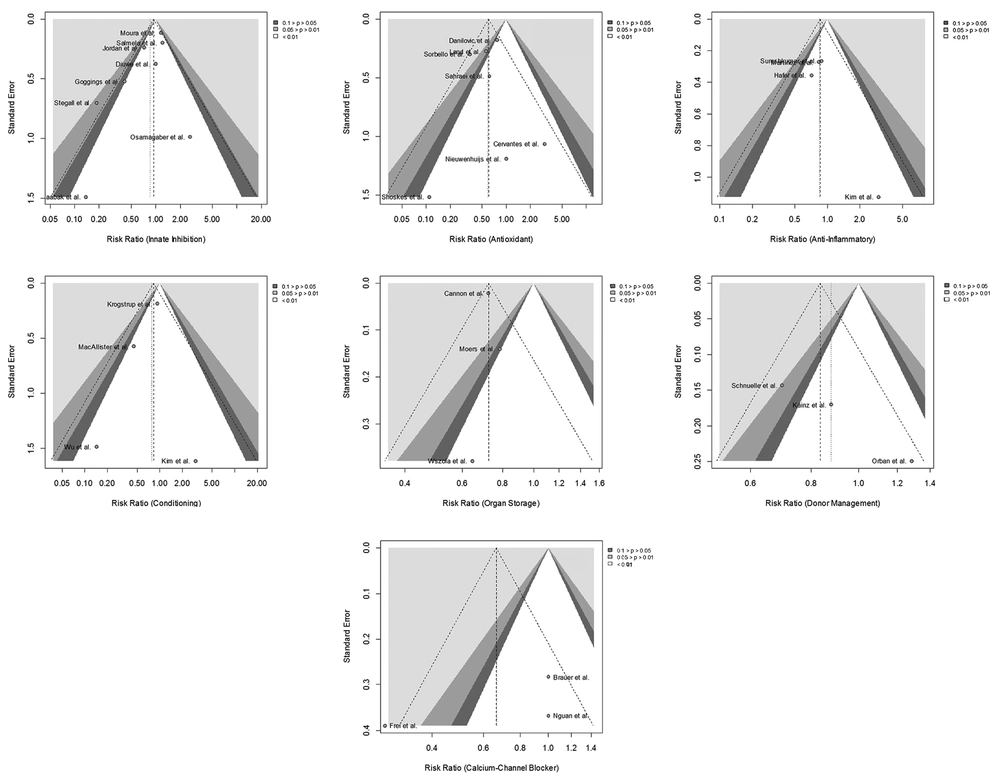
After the tabulation of the included studies, one urologist determined the category of the treatment hypothesis using one of the following terms: donor management, organ storage, anti-inflammatory treatment, antioxidant treatment, innate inhibition, calcium-channel blockers, and conditioning (15). The meta-analysis was conducted when relevant data were available from at least three studies in each category. If the study had more than one intervention or control group, both sample size and number of patients with events were summed across the groups (16). To quantify the heterogeneity between studies in each category, the I2 statistic and χ2 of the Cochran Q-test were calculated (17). The summary effect estimate (risk ratio (RR) and 95% confidence interval (CI)) of each prevention strategy was computed using the Mantel-Haenszel method with a continuity correction of 0.5 in studies with zero events (18). The fixed or random-effects model was used based on the level of heterogeneity. If a significant level of heterogeneity was detected (significant P value of Q-test or I2 ≥ 50), a random-effects model was used to calculate the summary effect estimate. Otherwise, a fixed-effects model was employed. To assess the publication bias in each category, a funnel plot was used to visualize the effect estimates (x-axis) against the standard errors (y-axis). Statistical analyses were performed using the “meta” package in the R studio (version 3.5.3, R Core Team, 2019).
3. Results
3.1. Search Results
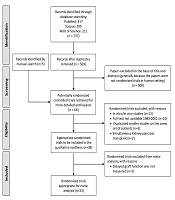
Of the 569 articles retrieved, 526 articles were excluded based on the screening of abstracts. A full-text review of the remaining 66 studies (five studies by manual search) resulted in the final inclusion of 38 studies in qualitative synthesis (Figure 1).
3.2. Characteristics of the Included Studies
Table 1 describes the key characteristics of the included RCTs. The studies were performed between 1990 and 2018, mostly conducted in the United States (21%). Eleven studies (28.9%) evaluated the effect of the oral or infusion administration of an antioxidant agent, mainly recombinant human Superoxide Dismutase (rh-SOD) and N-acetyl-cysteine (NAC) (19-29). Eight studies (21.1%) assessed the preventive effect of the innate inhibitors (e.g. eculizumab, thymoglobulin, etc.) (30-37). The effect of anti-inflammatory agents, including erythropoietin (EPO), was evaluated in six trials (38-43). Four studies evaluated a type of conditioning technique (i.e., remote ischemic (pre or post) conditioning) (44-47). Moreover, data on the evaluation of calcium-channel blockers (CCBs) (48-50), organ storage techniques (51-53), and donor management approaches (54-56) were separately available in three RCTs.
| Study | Year | Country or Region | Size (N) | Age | Male Gender | Category of Strategy | Agent or Technique | DGF (Int. vs. Con.) | Effectiveness | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Int. | Con. | Int. | Con. | Int. | Con. | |||||||
| Frei et al. (48) | 1990 | Germany | 65 | 64 | 41 ± 11 | 42 ± 12 | 39 (60) | 38 (59) | CCB | Diltiazem | 11% vs. 39%c | Yes |
| Pollak et al. (19) | 1993 | USA | 58 | 58 | 41.5 ± 1.6 | 41.5 ± 1.9 | 35 (60) | 43 (74) | Antioxidant | rh-SOD | NA | No |
| Rabl et al. (20) | 1993 | Austria | 14 | 16 | 41.56 ± 14.14 | 43 ± 9.86 | 22 (73.4) | Antioxidant | Omnibionta | NA | Yes | |
| Land et al. (21) | 1994 | Germany | 81 | 96 | 45.4 ± 12 | 45.6 ± 13 | 47 (58) | 67 (70) | Antioxidant | rh-SOD | 18.5% vs. 33.4%c | Yes |
| Salmela et al. (30) | 1999 | Europe | 131 | 131 | 48 | 45.1 | 83 (63.4) | 89 (67.9) | Innate Inhibition | Enlimomab | 31% vs. 26% | No |
| Goggins et al. (31) | 2003 | USA | 27 | 31 | 50 ± 12 | 51 ± 10 | 11 (41) | 16 (52) | Innate inhibition | Thymoglobulin | 14.8% vs. 35.5%c | Yes |
| Fontana et al. (22) | 2005 | Italy | 12 | 14 | 44 ± 13 | 44 ± 15 | 5 (41.7) | 10 (71.4) | Antioxidant | Fenoldopam | NA | No |
| Shoskes et al. (23) | 2005 | USA | 14 - 14 | 15 | 52.5-46 | 44 | 7 (50) - 7 (50) | 11 (71) | Antioxidant | Curcumin and quercetin | 0% - 0% vs. 14.3% | Yes |
| Basu et al. (24) | 2007 | Austria | 8 | 8 | 56.4 ± 9.5 | 11 (68.8) | Antioxidant | Propofol | NA | Yes | ||
| Nguan et al. (49) | 2008 | Canada | 23 | 23 | 48 ± 15 | 49 ± 13 | 13 (56.5) | 13 (56.5) | CCB | Verapamil | 39% vs. 39% | Yes |
| Moers et al. (51) | 2009 | Europe | 336 | 336 | 53 (11 - 79) | 52 (2 - 79) | NA | NA | Organ storage | Machine perfusion | 20.8% vs. 26.5%c | Yes |
| Schnuelle et al. (54) | 2009 | Europe | 227 | 260 | 52.8 ± 12.7 | 52.0 ± 12.4 | 133 (58.6) | 164 (63.1) | Donor management | Dopamine | 24.7% vs. 35.4%c | Yes |
| Kainz et al. (55) | 2010 | Austria-Hungary | 238 | 217 | 49.6 ± 14.4 | 49.2 ± 14.0 | 162 (68.1) | 140 (64.5) | Donor management | Methylprednisolone | 22% vs. 25% | No |
| Othman et al. (38) | 2010 | Egypt | 20 | 20 | 32 ± 9 | 34 ± 11 | 17 (85) | 17 (85) | Anti-inflammatory | Normal saline | 0% vs. 0% | Yes |
| Martinez et al. (39) | 2010 | France | 51 | 53 | 60 ± 7.7 | 58.9 ± 9.5 | 34 (66.7) | 30 (56.6) | Anti-inflammatory | EPO-β | 32% vs. 38.5% | No |
| Brauer et al. (50) | 2010 | Germany | 100 | 100 | 52 | 47 | 65 (65) | 66 (66) | CCB | Prostaglandin | 20% vs. 20% | No |
| Sorbello et al. (25) | 2010 | Italy | 40 - 40 - 40 | 20 | 56.4 ± 12.5 | 54.5% | Antioxidant | NAC | 22.5% - 7.5% - 22.5% vs. 50% | No | ||
| Danilovic et al. (26) | 2011 | Brazil | 38 | 36 | 50.7 ± 11.8 | 48.8 ± 11.1 | 13 (34.2) | 20 (55.6) | Antioxidant | NAC | 55.3% vs. 72.3%c | Yes |
| Gaber et al. (32) | 2011 | USA | 9 | 6 | 38 ± 13.2 | 54.7 ± 12.8 | 6 (66.7) | 5 (83.3) | Innate inhibition | YSPSL | 41% vs. 20% | No |
| Stegall et al. (33) | 2011 | USA | 26 | 51 | 48.6 ± 12.5 | 48.4 ± 11.5 | 5 (19.2) | 11 (21.6) | Innate inhibition | Eculizumab | 7.7% vs. 41%c | Yes |
| Hafer et al. (40) | 2012 | Germany | 44 | 44 | 53.6 ± 1.8 | 49.8 ± 1.6 | 25 (56.8) | 26 (59.1) | Anti-inflammatory | rHuEPO | 22.7% vs. 31.8% | No |
| Requiao-Moura et al. (34) | 2012 | Brazil | 97 | 112 | 45.2 ± 12.9 | 44.7 ± 14.4 | 49 (50.5) | 61 (54.4) | Innate inhibition | Thymoglobulin | 63.9% vs. 55.3% | Yes |
| Sureshkumar et al. (41) | 2012 | USA | 36 | 36 | 58 ± 11 | 56 ± 13 | 19 (53) | 20 (56) | Anti-inflammatory | EPO-α | 41.7% vs. 47.2% | No |
| Kim et al. (42) | 2013 | South Korea | 30 | 30 | 44 ± 12 | 46 ± 12 | 17 (56.7) | 21 (70) | Anti-inflammatory | Plasmalyte | 10% vs. 3.4% | Yes |
| Ojeda-Cervantes et al. (27) | 2013 | Mexico | 10 | 10 | 31.5 (24.8 - 37.5) | 32 (23.3 - 43.8) | 4 (40) | 6 (60) | Antioxidant | Spironolactone | 30% vs. 10% | Yes |
| Cannon et al. (52) | 2013 | USA | 13293 | 13293 | 53.4 ± 12.8 | 53.4 ± 12.9 | NA | NA | Organ storage | Machine Perfusion | 21% vs. 29%c | Yes |
| Kim et al. (44) | 2014 | South Korea | 30 | 30 | 42 (34 - 52) | 44 (33 - 51) | 15 (50) | 11 (36.7) | Conditioning | RiPoC | 3% vs. 0% | No |
| Wszola et al. (53) | 2014 | Poland | 16 - 10 | 9 - 27 | 50 ± 16 - 39.7 ± 10 | 53.1 ± 8 - 41.6 ± 12 | 8 (50) - 6 (60) | 5 (56) - 16 (59) | Organ storage | Machine Perfusion | 31% - 20% vs. 56% - 37% | Yes |
| Wu et al. (45) | 2014 | China | 24 | 24 | 40.6 ± 11.6 | 39.7 ± 10.2 | 12 (50) | 15 (62.5) | Conditioning | RiPoC | 0% vs. 13% | Yes |
| MacAllister et al. (46) | 2015 | Europe | 102 - 103 - 102 | 99 | 47.6 ± 15.1-45.9 ± 14.2-45.3 ± 15.3 | 46.8 ± 15.1 | 71 (72.4)-65 (65.7) - 73 (73.7) | 58 (61.1) | Conditioning | RIPC | 2.4% vs. 5.4% | No |
| Orban et al. (56) | 2015 | France | 82 | 78 | 44.6 ± 15.5 | 50.5 ± 13.3 | 51 (62) | 56 (76) | Donor management | NAC | 33% vs. 25.4% | No |
| Sahraei et al. (28) | 2015 | Iran | 33 - 19 | 32 | 37.23 ± 16.53-36.90 ± 16.71 | 36.17 ± 17.06 | 16 (49) - 12 (63) | 20 (63) | Antioxidant | NAC | 12.1% - 15.8% vs. 21.9% | No |
| Hatem et al. (43) | 2017 | UK | 77 | 63 | 47 ± 11.7 | 42 ± 11.4 | 53 (68.8) | 39 (61.9) | Anti-inflammatory | Aspirin | NA | No |
| Diuwe et al. (35) | 2017 | Poland | 47 | 47 | 47.1 ± 14 | 51.9 ± 13.8 | 33 (70.2) | 32 (68.1) | Innate inhibition | Etanercept | 23.4% vs. 23.4% | No |
| Krogstrup et al. (47) | 2017 | Europe | 109 | 113 | 58.1 (49.5 - 65.0) | 61.4 (49.4 - 66.6) | 65 (60) | 69 (61) | Conditioning | RIC | 33% vs. 35% | No |
| Nieuwenhuijs-Moeke et al. (29) | 2017 | Netherlands | 20 - 18 | 19 | 51.5 ± 10.4 - 48.8 ± 15.4 | 52 ± 11.5 | 8 (40) - 11 (61) | 8 (42) | Antioxidant | Propofol | 0% - 11% vs. 5% | Yes |
| Jordan et al. (36) | 2018 | USA | 35 | 35 | 57.66 ± 7.74 | 58.14 ± 11.18 | 20 (57.14) | 22 (62.86) | Innate inhibition | C1-INH | 44% vs. 60% | No |
| Kaabak et al. (37) | 2018 | Russia | 29 | 28 | 7.6 ± 5.2 | 8.3 ± 3.9 | 16 (55.2) | 14 (50) | Innate inhibition | Eculizumab | 0% vs. 10.7% | Yes |
Abbreviations: CCB, calcium-channel blocker; C1-INH, C1-inhibitor; Con., control; DGF, delayed graft function; eGFR, estimated glomerular filtration rate; EPO-α, erythropoietin alpha; EPO-β, erythropoietin beta; Int., intervention; NAC, N-acetyl-cysteine; rh-SOD, recombinant human superoxide dismutase; rHuEPO, recombinant human erythropoietin; RIC, remote ischemic conditioning; RIPC, remote ischemic preconditioning; RiPoC, remote ischemic post-conditioning; UK, United Kingdom; USA, the United States of America; YSPSL or rPSGL-Ig, recombinant P-selectin glycoprotein ligand IgG fusion protein.
aThe intervention was considered effective, if P value was significant for at least one outcome.
bThe values are expressed as mean ± SD, median (IQR), or No. (%).
cThe primary outcome, DGF, was significantly different between intervention and control groups.
Around 47.4% of the studies did not reach a statistically significant level for any of their defined primary or secondary outcomes. A total of 28 (73.7%) studies included deceased donor recipients, eight (21.1%) studies employed living donor recipients, and two studies combined both deceased and living donor recipients (37, 53). Only one study enrolled pediatric kidney recipients (age ≤ 18 years) (37).
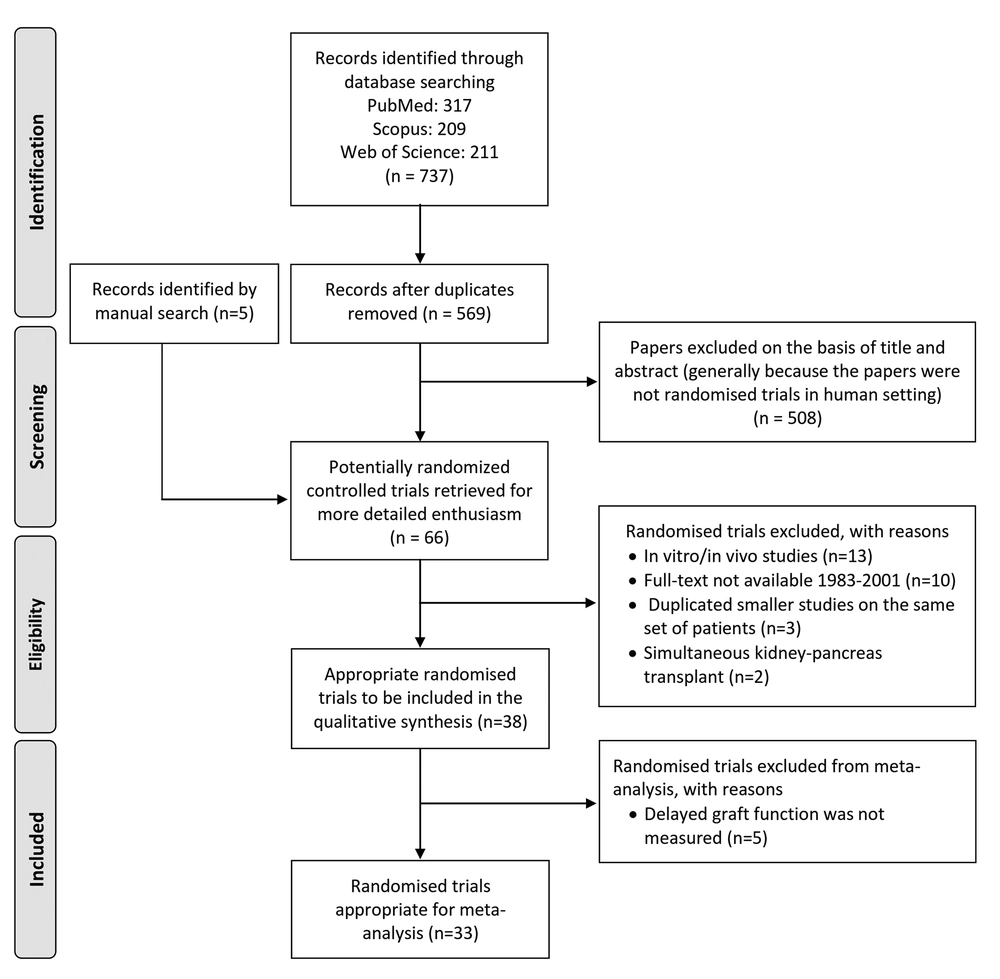
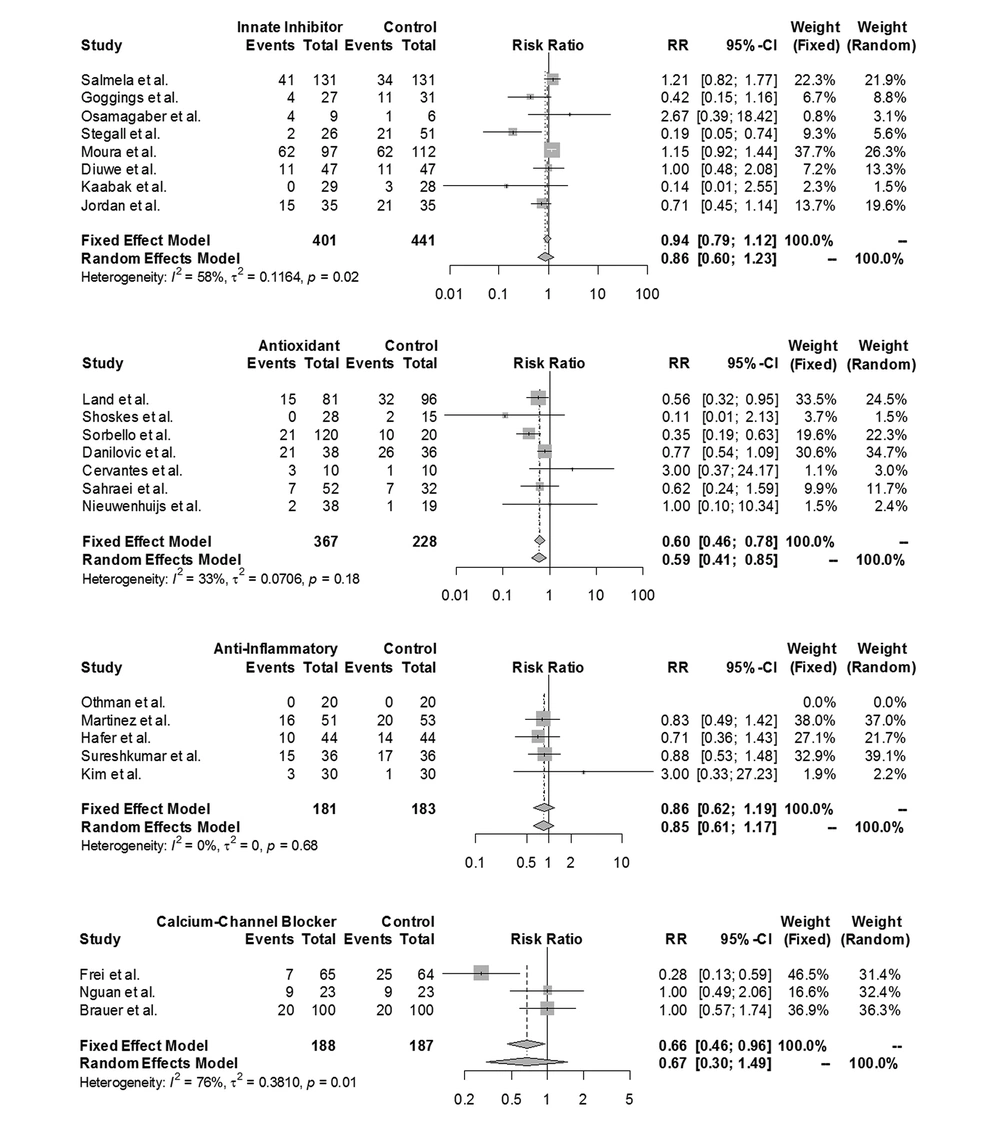
Five studies were excluded from the meta-analysis since they did not report the DGF rate after the study period. The remaining 33 studies included a total of 31,334 patients (15,809 in intervention and 15,525 in control groups). As shown in Figures 2 and 3, of the patients in the intervention groups, 13,655 patients received kidneys stored with machine perfusion rather than preserved in cold storage, 547 patients received grafts from donors who were prepared with an additional preventive agent, 470 patients experienced a type of conditioning technique before or during the surgery, 401 patients received innate inhibitors, 367 patients received antioxidants, 188 patients received CCBs, and 181 patients received anti-inflammatory agents.
3.3. Risk of Bias and Quality Assessment
The risks of selective reporting and attrition bias were deemed unclear for the majority of the studies (87% and 79%, respectively). A total of 41 studies were associated with an unclear or high risk of selection bias due to the insufficient description of random sequence generation or allocation concealment. The performance and detection bias issues were fully explained in 50% and 58% of the studies, respectively. According to the AHRQ guidelines, about 8%, 16%, and 86% of the studies were considered methodologically to be of the good, fair, and poor level of quality, respectively (Table 2).
| Study | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Selective Reporting (Reporting Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Other Bias | AHRQ Quality |
|---|---|---|---|---|---|---|---|---|
| Frei et al. (48) | U | H | U | U | U | U | U | Poor |
| Pollak et al. (19) | L | L | U | L | L | U | L | Fair |
| Rabl et al. (20) | U | U | U | U | U | U | U | Poor |
| Land et al. (21) | U | U | U | L | L | U | U | Poor |
| Salmela et al. (30) | U | U | U | U | L | U | L | Poor |
| Goggins et al. (31) | U | U | U | U | U | U | U | Poor |
| Fontana et al. (22) | U | U | U | U | U | U | U | Poor |
| Shoskes et al. (23) | U | U | U | L | L | U | L | Poor |
| Basu et al. (24) | U | U | U | U | U | U | U | Poor |
| Nguan et al. (49) | H | H | U | H | H | U | U | Poor |
| Moers et al. (51) | L | L | U | U | U | U | L | Poor |
| Schnuelle et al. (54) | L | H | U | L | L | U | L | Poor |
| Kainz et al. (55) | L | L | L | L | L | L | L | Good |
| Othman et al. (38) | L | L | U | L | H | U | L | Poor |
| Martinez et al. (39) | U | H | U | L | L | L | L | Poor |
| Brauer et al. (50) | U | U | U | U | U | U | U | Poor |
| Sorbello et al. (25) | U | U | U | U | U | U | L | Poor |
| Danilovic et al. (26) | L | L | U | L | L | U | L | Fair |
| Gaber et al. (32) | L | U | U | L | L | L | L | Fair |
| Stegall et al. (33) | U | U | U | U | L | U | H | Poor |
| Hafer et al. (40) | L | L | U | L | L | L | H | Poor |
| Requiao-Moura et al. (34) | H | H | U | U | U | U | U | Poor |
| Sureshkumar et al. (41) | L | L | U | L | L | U | L | Poor |
| Kim et al. (42) | L | L | U | L | L | U | L | Poor |
| Ojeda-Cervantes et al. (27) | U | U | U | U | U | U | U | Poor |
| Cannon et al. (52) | L | U | U | U | L | L | H | Poor |
| Kim et al. (44) | L | L | H | L | L | U | L | Poor |
| Wszola et al. (53) | U | U | U | U | U | U | U | Poor |
| Wu et al. (45) | U | U | U | U | L | U | U | Poor |
| Orban et al. (56) | L | L | U | L | L | U | L | Fair |
| MacAllister et al. (46) | L | L | L | L | L | L | L | Good |
| Sahraei et al. (28) | L | L | U | U | U | L | L | Poor |
| Hatem et al. (43) | H | H | U | U | U | U | H | Poor |
| Nieuwenhuijs-Moeke et al. (29) | L | L | U | L | L | U | L | Fair |
| Krogstrup et al. (47) | L | L | L | L | L | L | L | Good |
| Diuwe et al. (35) | U | U | U | U | U | U | U | Poor |
| Jordan et al. (36) | L | L | H | L | L | U | L | Poor |
| Kaabak et al. (37) | L | L | U | L | L | U | L | Fair |
Abbreviations: AHRQ, Agency for Healthcare Research and Quality; H, high; L, low; U, unclear.
The inspection of the funnel plots represented remarkable asymmetry in four categories, suggesting a high level of publication bias. The innate inhibition, antioxidant, and conditioning categories were found to have a fair level of publication bias (Figure 4).
3.4. Risk Ratio of Delayed Graft Function
The fixed-effects meta-analysis suggested a trend toward machine perfusion in reducing the DGF rate in comparison with the traditional cold storage method (RR = 0.73; 95% CI = 0.7 to 0.76; I2 = 0%; P = 0.81). Moreover, a statistically significant reduction in DGF was observed when an antioxidant agent was used (RR = 0.6; 95% CI = 0.46 to 0.78; I2 = 33%; P = 0.18).
No significant effect was demonstrated for innate inhibitors (RR = 0.86; 95% CI = 0.6 to 1.23; I2 = 58%; P = 0.02), anti-inflammatory agents (RR = 0.86; 95% CI: 0.62 to 1.19; I2=0%; P=0.68), CCBs (RR=0.67; 95% CI = 0.3 to 1.49; I2 = 76%; P = 0.01), conditioning (RR = 0.83; 95% CI = 0.59 to 1.16; I2 = 16%; P = 0.31), and donor management techniques (RR = 0.88; 95% CI = 0.64 to 1.2; I2 = 57%; P = 0.1). The forest plots of the prevention categories are shown in Figures 2 and 3.
3.5. Adverse Events
Eighteen studies specified the occurrence of adverse reactions to prevention strategies. Of these, eight studies observed infection, mainly as a result of using innate inhibitors or CCBs (e.g., enlimomab, eculizumab, and thymoglobulin) (27, 30, 31, 33, 34, 37, 44, 50). The cytomegalovirus (CMV) and urinary tract infection (UTI) were the most frequent infections. In one study, the number of patients with CMV infection was significantly higher in the thymoglobulin group than in the control group (58.3% vs. 17.1%; P < 0.001) (34). There was also one incidence of Burkitt’s lymphoma in the eculizumab group that resulted in the patient’s death after deciding not to receive chemotherapy treatment (33). Four episodes of flu-like infection were observed in pediatric patients receiving eculizumab (37).
Five studies reported circulatory adverse events, including hypotension and tachycardia (25, 41, 48, 49, 54). Moreover, a total of 171 out of 307 patients developed paraesthesia or skin petechiae during remote ischemic preconditioning (RIPC) (46). A malfunctioning machine also caused a serious event due to uninterrupted inflation of the cuff during the conditioning process (47).
4. Discussion
Using the expanded criteria organs increases the risk of IRI, which diminishes the short- and long-term allograft function after kidney transplantation (57). Since the exact cellular mechanism of IRI is not fully understood, numerous studies have been performed focusing on different prevention hypotheses, which have been successfully tested in animal studies (58-61). However, our understanding of the utility and application of these strategies in the human setting is limited with no previously published comprehensive systematic review. Fixed and random-effects meta-analyses of the currently reported literature suggested that using the machine perfusion organ storage (RR = 0.73; 95% CI = 0.7 to 0.76) and administration of antioxidant agents (RR = 0.6; 95% CI = 0.46 to 0.78) significantly reduce the risk of DGF. The overall quality of evidence was low for the majority of the included studies (86%) based on the AHRQ definitions.
The fixed-effects model of three homogenous studies in the organ storage category showed that although machine perfusion of donated kidneys is associated with longer cold ischemia time, it appears to offer remarkable protection against DGF (51-53). Two recent reviews confirmed the advantages of the hypothermic machine perfusion against IRI (62, 63). Moreover, it has been previously shown that the use of machine perfusion is associated with prolonged graft survival rate compared to the usual cold storage method (63).
Seven studies evaluating the effect of five antioxidants (i.e., rh-SOD, NAC, spironolactone, propofol, and curcumin) showed that the attenuation of oxidative stress improved sustained recovery of graft function. It has been previously shown that NAC has beneficial effects on the pathogenesis of acute renal disorders, including ischemic injuries (64, 65). Moreover, NAC preserves renal function during ischemic or reperfusion episodes by reducing renal interstitial inflammation. These effects are connected with increased renal glutathione levels, showing that NAC weakens renal oxidative stress (66).
Most of the studies on the preventive effect of rh-SOD were performed in animal models in the 1990s (67). The hypothesis is that superoxide dismutase has beneficial effects on free radical-induced injuries as a powerful scavenger of superoxide anions. In addition, SOD catalyzes the dismutation of superoxide to hydrogen peroxide without affecting other parts of the molecular environment. This mechanism results in scavenging oxygen free radicals generated by hypoxia at the molecular level (68). Other antioxidants, including curcumin and spironolactone, have been evaluated focusing on the same mechanism. The advantageous effect of spironolactone on IRI has been previously confirmed in a rat model (69), but to the best of our knowledge, there are no similar studies on the preventive effect of curcumin in the transplantation area.
It is evident that the complex interplay of the innate and adaptive immune responses contributes to the pathogenesis of cell-mediated and antibody-mediated rejections (70). The following mechanisms and cells can contribute to immune response: cells (e.g., neutrophils, macrophages, dendritic cells or DCs, etc.), Toll-like receptors (TLRs), and the complement cascade.
It has been previously shown that the rabbit anti-thymocyte globulin (rATG) or thymoglobulin inhibits the function of DCs (71). A recent study in mice model has confirmed the powerful protective effect of T cell-specific NF-κB against IRI (72). Goggins et al. (31) and Requiao-Moura et al. (34) sought to study the effect of intraoperative administration of 3 - 6 and one dose(s) of Thymoglobulin, respectively (1.5 and 1 mg/kg). Contrary to the former study, Requiao-Moura et al. (34) showed that the incidence rate of DGF was similar in both groups (P = 0.36). The important characteristic of this study was the prolonged mean cold ischemia time (24 h) compared with the former study (13 h). It might be noted that the immunoprophylaxis effect of thymoglobulin might be suppressed in case of prolonged cold ischemia time. Moreover, the recombinant P-selectin glycoprotein ligand-Ig fusion protein (rPSGL-Ig) prevents granulocyte adhesion by binding the P- and E-selectin. Gaber et al. showed that YSPSL did not affect the DGF rate. However, treated patients had significantly lower creatinine levels (32). Further studies are needed to define the effect of YSPSL on early renal allograft function.
Several studies tried to inhibit the complement cascade as an important component of the innate immune response. The complement activation results in renal damage in two phases: first, during reperfusion after the cold ischemia time and second, after donor antigens are recognized by the adaptive immune system. Eculizumab is a long-acting, recombinant, humanized anti-C5 monoclonal antibody that might be useful as a prophylactic agent for the prevention of IRI. The infusion of eculizumab before graft reperfusion resulted in a lower DGF rate in the intervention group in two trials (33, 37). However, only one study reached a statistically significant level (33). Since it takes some hours to see the suppressive effect of eculizumab, it should be noted that pre-transplant infusion just before graft perfusion might reduce its effectiveness. Moreover, the beneficial effect of C1-inhibitor (C1-INH) also has been mostly observed in rodents (73). The only trial included in our review confirmed its preventive effect by blocking the classical or lectin pathway of the complement cascade (36).
Previous studies in animal models have shown encouraging results on the protective effect of EPO against IRI (74, 75). The hypothesis is that when EPO receptors are activated, EPO leads to a protective effect on proximal tubular epithelial cells. In contrast to the promising findings of animal studies, the included trials in human settings showed a significant reduction neither in the incidence rate of DGF nor in graft function. However, since confidence intervals are rather large, the possibility of the significant effect of EPO in larger populations cannot be definitely excluded.
Ischemia causes the intracellular accumulation of Na+ ions and Ca+ levels. This calcium overload results in the activation of calcium-related proteases. Calpains are one of these proteases that remains inactive due to acidosis environment during the ischemic phase. After pH normalization at reperfusion, calpains cause cell structure impairment and consequently cell death. The Ca+ accumulation also causes the generation of reactive oxygen species (ROS). Calcium-channel blockers are used to slow down this harmful process. However, currently published articles did not show the protective effect of verapamil (organ reperfusion) and prostaglandin (infusion) against IRI (49, 50). In contrast, Frei et al. (48) showed that the intraoperative infusion of diltiazem in addition to diltiazem pretreatment (organ reperfusion in 500 ml Euro-Collins with diltiazem) significantly decreased the DGF rate.
The protective effect of remote ischemic conditioning (RIC) has been shown in a variety of clinical settings (e.g. heart and liver) (76). Four studies performed 3 - 4 cycles of 5-min repetitive ischemia and reperfusion by clamping either upper limb or thigh before graft reperfusion (44-47). None of the studies found significant differences in the DGF rate between the control and intervention groups. However, the secondary analysis of the large REPAIR (REmote preconditioning for protection against ischemia-reperfusion in renal transplantation) study found a significant improvement in eGFR (P = 0.022) (46). Considering the low cost of delivery, no potential harms, and the chance of improving graft function, future investigations are required to define the role of RIC in both deceased and living donor transplantation areas.
Another important factor to reduce the risk of IRI is the optimal management of deceased donors. A published article in 2015 showed that donor pretreatment with NAC did not reduce the DGF rate, as well as short-term graft function assessed by serum creatinine, eGFR, and urine output (56). Moreover, Kainz et al. (55) did not observe a significant improvement in graft function after administration of 1,000 mg corticosteroids to donors at least three hours before organ harvesting. In contrast, a randomized, multi-center trial in Europe showed the salutary effect of donor pretreatment with low-dose dopamine on graft performance (54).
4.1. Limitations and Strengths
The following limitations should be acknowledged both at qualitative and quantitative levels. First, a high degree of heterogeneity in innate inhibitor, CCB, and donor management groups prevented us to fully assess the effect of these strategies on the DGF rate. This heterogeneity could possibly be due to high variation among included studies concerning the ethnicity of participants, administration time of the preventive agent (e.g., before, during, or after transplantation), mode of administration (e.g., infusion, oral, etc.), the dosage of the agents, etc. However, we could not perform a meta-regression analysis due to the paucity of the relevant data. Second, we extracted the DGF rates based on the definitions used in the trials. As suggested by a previously published systematic review, this heterogeneity in DGF definitions hinders the evolution of both clinical and research practices for the diagnosis and treatment of IRI (8). Third, our qualitative synthesis showed that the published trials were mostly of fair or poor quality level. Fourth, since most of the included trials had small-sized populations, the results represented small effect estimates, which may affect the generalizability of the findings.
Despite the limitations, our review included randomized controlled trials from 18 countries, which included diverse practice settings from different developing and developed countries. Moreover, a fixed-effects meta-analysis was carried out in four categories, which showed the acceptable level of homogeneity within the included trials in these groups.
5. Conclusions
Considering the evidence on the reduced DGF rate by the machine perfusion storage technique, it might be proposed to substantially expand the use of machine perfusion beyond current levels. In contrast, high adverse events of innate inhibitors in addition to their low effectiveness highlight the need for large-scale randomized controlled trials to be conducted in a clinically useful manner. Moreover, using the standard definition of DGF will help future researchers to integrate the results and provide practical guidelines.