1. Background
End-stage renal disease (ESRD) is the end stage of chronic kidney disease (CKD), which requires renal replacement therapy. Its prevalence is continuously increasing so that according to guesstimates, over 500 million people are susceptible to develop ESRD worldwide. The prevalence and incidence of ESRD in the Middle East countries range from 818 per million population (pmp) in Lebanon to 55 pmp in Iraq, and 49.9 pmp in Iran to 276 pmp in Turkey (1). Its treatment costs, morbidity, and mortality are high (1-3). Cardiovascular diseases are the most common causes of death in these patients. They have many traditional and non-traditional risk factors, such as malnutrition, oxidative stress, and inflammation, which may be more important than others (4). In CKD, when GFR decreases, the inflammation increases. Multiple mechanisms are needed to explain the causes of inflammation, such as decreased cytokines elimination, frequent infections, oxidative stress, intestinal dysbiosis, periodontal disease, metabolic acidosis, vitamin D deficiency, and dialysis-related factors (5).
Different biomarkers with different predictive values may increase in patients with CKD/ESRD. One of the most important ones is C-reactive protein (CRP), which is associated with underlying inflammation and generation of proinflammatory cytokines, such as interleukin (IL)-6 and tumor necrosis factor-a (TNF-a), which can predict mortality (6, 7). Albumin is used for the assessment of nutritional status. Cardiovascular diseases are the most common causes of death in these patients. Inflammation and malnutrition can result in carotid atherosclerosis and cardiac mortality (5). Besides, these factors increase all-cause mortality (6).
Coenzyme Q10 (CoQ10), with a benzoquinone structure, has multiple effects on the metabolism, including the synthesis of adenosine triphosphate (ATP), which is a critical intermediate mediator of mitochondrial calcium-dependent ion channels. Others can be effective in antioxidant activities, free radical scavenging, and restoration of the antioxidant defense system (8, 9).
2. Objectives
Since a few studies have been conducted in this field and the effects of CoQ10 in ESRD and inflammatory processes are less studied, the current study investigated the effects of CoQ10 therapy on CRP, albumin, and homocysteine in hemodialysis patients in Zahedan.
3. Method
Forty adults with diabetes and ESRD, receiving hemodialysis for at least six months and having kidney failure following diabetes mellitus, were enrolled in this double-blinded randomized clinical trial study. Participants were selected from the hemodialysis unit of Ali-Ibne Abitaleb and Khatamolanbia hospitals of Zahedan from April 2015 to July 2016. In total, 200 patients were evaluated, 40 of whom had the inclusion criteria. They all were taking folic acid in the past six months. Patients were excluded if they had uncontrolled hypertension, history of smoking, active infection, liver disease, autoimmune disease, age of fewer than 18 years, malignancies, and recent hospitalization, or were taking vitamin C, vitamin E, L-carnitine, or other supplements. The study was approved by the Ethics Committee (IR.zaums.rec.1395.235 and IRCT code: IRCT2017060734370n1).
First, the study was described for patients, and they were told that they are free to leave the study whenever they want. Then, if they were agreed, informed consent was taken from them. The investigated variables were age, gender, blood pressure, serum albumin, CRP, Kt/V, and homocysteine. In all participants, Kt/V was > 1.2, and blood pressure was below 160/100 mm/Hg in the past three months. Patients were randomly divided into two groups. The treatment group included 20 patients who received a supplement of coenzyme Q10 (21ST century, USA), 200 mcg once daily. The control group included 20 patients who received a placebo for 24 weeks. Then, albumin, CRP, and homocysteine of the participants were rechecked. Data were reported as the mean ± standard deviation (SD). To check the normality of distribution, the Kolmogorov-Smirnov test was used. For data with normal distribution, the independent or paired sample t test was used to compare the means or ranks between or within the groups, respectively. For other types of data, the Mann-Whitney and Wilcoxon signed-rank tests were used. Data were analyzed using SPSS v.17 software (IBM Analytics, USA). A P-value < 0.05 was considered statistically significant.
4. Results
Forty patients on dialysis at two hospitals were studied. Twelve (60%) females and 11 (55%) females were in Q10 and placebo groups, respectively. The mean age of the patients was 60 ± 9 years. Sex distribution and the mean age of the patients did not show significant differences between the groups (P > 0.05).
Before and after the study, the albumin level did not show any significant difference between the groups; however, the mean rank of albumin changes was significant between the groups (Table 1) and the albumin level decreased in the placebo group after the intervention. To compare albumin before and after the intervention in each group separately, the Wilcoxon signed-rank test was used. The results showed no significant difference in the albumin levels before and after the intervention in each group (P > 0.05).
| Baseline Characteristics | Total | Q10 | Placebo | P Value |
|---|---|---|---|---|
| Age (years) | 60.3 ± 8.3 | 60.2 ± 8.5 | 60.4 ± 8.2 | 0.96 |
| Gender | 0.78 | |||
| Male | 17 (42.5) | 9 (45) | 8 (40) | |
| Female | 23 (57.5) | 11(55) | 12 (60) | |
| Dialysis (Hours/week) | 12 | 12 | 12 | |
| Kt/V | 1.2 ± 0.20 | 1.2 ± 38 | 1.2 ± 12 | 0.86 |
aValues are expressed as mean ± SD and No. (%).
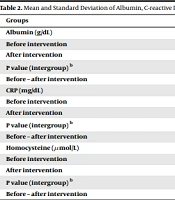
C-reactive protein did not show any difference between or within the groups before and after the intervention. This result was found for delta CRP before and after the intervention, too (Table 2). Besides, the comparison of case and control groups showed that the mean homocysteine levels before or after the intervention, as well as delta homocysteine variations, were not significant (Table 2). To compare the homocysteine changes before and after the intervention in each group separately, the Wilcoxon signed-rank test was used. The results showed no significant difference before and after the intervention in each group (P > 0.05).
| Groups | Q10 | Placebo | P Value (Between Group) a |
|---|---|---|---|
| Albumin (g/dL) | |||
| Before intervention | 4.03 ± 0.37 | 4.27 ± 0.33 | 0.24 |
| After intervention | 4.08 ± 0.23 | 3.82 ± 0.82 | 0.71 |
| P value (intergroup) b | 0.95 | 0.10 | |
| Before – after intervention | -0.05 ± 0.40 | 0.45 ± 0.80 | 0.08 |
| CRP (mg/dL) | |||
| Before intervention | 6.66 ± 7.05 | 13.75 ± 18.27 | 0.32 |
| After intervention | 9.58 ± 14.25 | 13.91 ± 16.32 | 0.8 |
| P value (intergroup) b | 0.35 | 0.67 | |
| Before – after intervention | -2.91 ±8.39 | 3.25 ± 13.84 | 0.22 |
| Homocysteine (µmol/L) | |||
| Before intervention | 32.75 ± 16.78 | 27.66 ± 9.69 | 0.51 |
| After intervention | 28.83 ± 12.71 | 26.05 ±7.23 | 0.84 |
| P value (intergroup) b | 0.25 | 0.42 | |
| Before – after intervention | 3.91 ±9.86 | 1.66 ± 9.01 | 0.97 |
a Based on Mann-Whitey test
bWilcoxon signed-rank test
5. Discussion
The prevalence of end-stage renal disease (ESRD) is increasing all around the world (9). In Iran, with more than 13,000 dialysis patients each month, 150,000 dialysis sessions are performed (10). End-stage renal disease increases inflammation, which, in turn, increases mortality from cardiovascular diseases (5). Antioxidants such as coenzyme Q10 can affect this process and reduce serum CRP as an inflammatory marker. In the current study, 57.5% of the participants were female, and the mean age was 60.3 ± 9.1 years. According to the results, although the albumin level slightly increased in the CoQ10 group and decreased in the placebo group, CoQ10 had no significant effect on the albumin level.
Also, CoQ10 did not have any significant effect on CRP; that is, the CRP level did not change significantly in either the CoQ10 or placebo group. The results on albumin are different from the results reported by Zahed et al., in which the authors reported that Co-Q10, 100 mg daily, significantly increased the albumin level after the intervention while CRP decreased (10). Zhai et al., in a systematic review and meta-analysis, reported that Co-Q10 supplementation had no effect on CRP, and various factors such as duration of the study, the dosage of Co-Q10, and sample size may affect the null effect on CRP (11). Raygan et al. evaluated the use of CoQ10 supplementation in diabetic or obese patients and reported no significant effect on total antioxidant capacity, as well as no effect on inflammatory markers such as CRP (12). Lee et al. (2013) investigated the effect of 300 mg/day of CoQ10 supplement on inflammation and oxidation and reported that this supplement had a better antioxidant effect on patients with coronary artery disease, but they noted that this intervention was not effective in reducing CRP. They concluded that pro-inflammatory cytokines such as IL-6 and TNF-a were more sensitive than CRP in inflammatory reactions (13). In another systematic review, Fan et al. concluded that although the level of CRP was reduced, the results should be interpreted with caution because few studies were done (14). Besides, for hemodialysis patients, particularly diabetic patients, the level of CRP was significantly higher, which could be attributed to the decreased response (15).
The results of this study showed that the supplementation with CoQ10 did not have any significant effect on the level of homocysteine. There was a slight decrease in both CoQ10 and placebo groups, but their difference was not statistically significant. The results are consistent with the study by Zahed et al. that reported the effect of Co-Q10 on the level of homocysteine was not significant (10). However, Ritu et al. reported that the use of this supplement significantly reduced the level of homocysteine in patients with cardiac disease (16). In general, the results of various studies performed on the effects of Q10 on the homocysteine level are contradictory, which can be attributed to differences in the demographic characteristics of participants, including age, gender, race, background diseases, the supplement dose of Q10, and its duration, and the type of drugs used by the patient. For example, vitamin B6 is necessary for the synthesis of CoQ10, and a study reported that the co-administration of coenzyme Q10 and B vitamins not only results in the better synthesis of endogenous coenzyme Q10 but also reduces the homocysteine levels (16, 17). The effects of coenzyme Q10 in dialysis patients may be associated with the higher levels of inflammatory markers in these patients than in healthy people. Since studies conducted in this area are contradictory and limited, it is suggested that more studies with larger sample sizes and longer follow-ups be conducted so that more firm conclusions can be drawn on the effects of Q10 on inflammatory factors, particularly homocysteine levels.
5.1. Conclusion
The results of this study showed that the supplementation with coenzyme Q10 did not affect the factors studied including albumin, CRP, and homocysteine.
5.2. Suggestions for Future Studies
Considering that studies are limited on the effects of coenzyme Q10 supplementation on the level of albumin, CRP, and homocysteine, and the results of these studies are contradictory, it is suggested that studies be done with larger sample sizes and longer duration of follow-up.